
(C-Cl) bond in $C{{H}_{2}}=CH-Cl$ (vinyl chloride) is stabilised in the same way as in:
a.) Benzyl chloride
b.) Ethyl chloride
c.) Chlorobenzene
d.) Allyl chloride
Answer
563.1k+ views
Hint: When we draw two or more than two Lewis dot structures for a molecule, which differs only in the locations of the electrons then we draw the resonance structure for those molecules. The actual structure is the resonance hybrid of all of the structures.
Complete step by step answer:
- Lewis dot structures also known as Lewis electron-dot formulas. It uses dots arranged around the chemical symbol for an element to represent the valence electron configuration of the atoms in the element. we should follow the octet rule in filling electrons in the outer orbital. So, we will form covalent bonds between two atoms which are formed by sharing electrons from one electron from each atom. The resonance structures are made by the delocalisation of electrons.
- Vinyl chloride i.e. $C{{H}_{2}}=CH-Cl$ contains pi electrons due to which vinyl chloride shows resonance. Thus, we can say that the C-Cl bond in vinyl chloride is stabilised by resonance.
Similarly, in chlorobenzene, due to resonance the bond in the compound is stabilized.
The resonating structure of vinyl chloride is mentioned below:
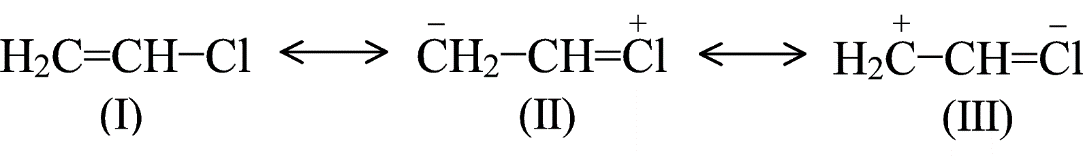
The correct option is option “C” .
Note: In general, when we have to solve the question from organic chemistry be careful with the inductive and resonance effect as in many of the cases resonance effect is stronger and in many cases inductive effect is stronger as compared to resonance effect.
Complete step by step answer:
- Lewis dot structures also known as Lewis electron-dot formulas. It uses dots arranged around the chemical symbol for an element to represent the valence electron configuration of the atoms in the element. we should follow the octet rule in filling electrons in the outer orbital. So, we will form covalent bonds between two atoms which are formed by sharing electrons from one electron from each atom. The resonance structures are made by the delocalisation of electrons.
- Vinyl chloride i.e. $C{{H}_{2}}=CH-Cl$ contains pi electrons due to which vinyl chloride shows resonance. Thus, we can say that the C-Cl bond in vinyl chloride is stabilised by resonance.
Similarly, in chlorobenzene, due to resonance the bond in the compound is stabilized.
The resonating structure of vinyl chloride is mentioned below:
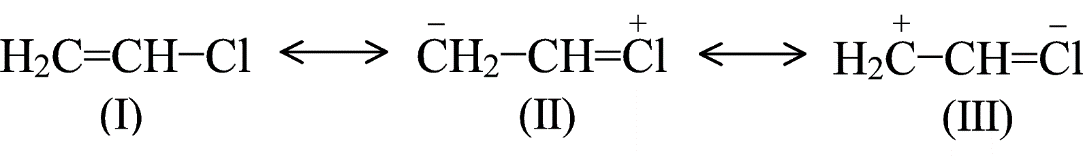
The correct option is option “C” .
Note: In general, when we have to solve the question from organic chemistry be careful with the inductive and resonance effect as in many of the cases resonance effect is stronger and in many cases inductive effect is stronger as compared to resonance effect.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




