
Cellulose is made up of :-
A. B-glucose and b galactose
B. Glucose and mannose
C. Linear polymer of b-D glucose unit
D. Linear polymer of N-acetyl D-glucosamine
Answer
607.2k+ views
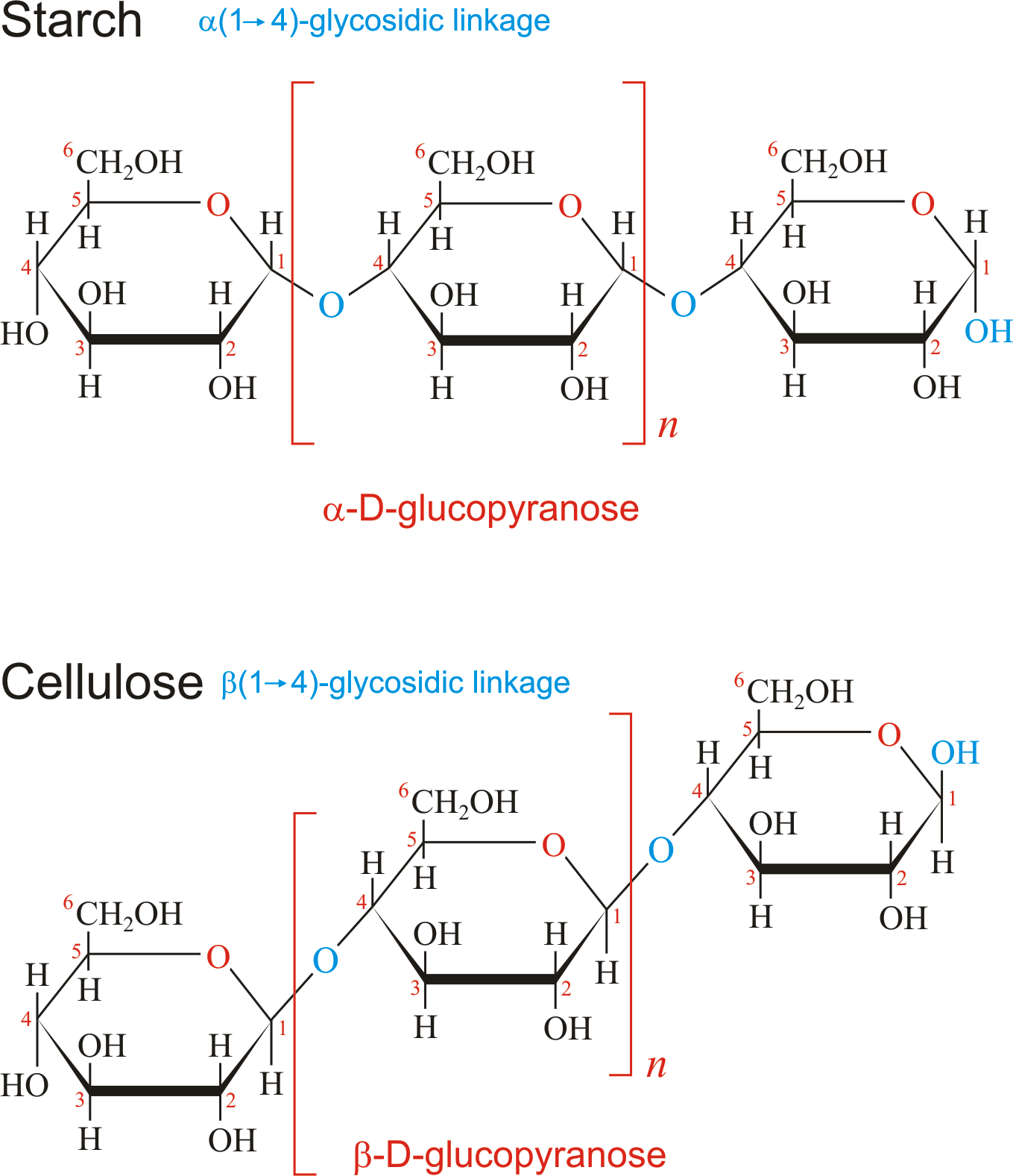
Hint : Cellulose is a polysaccharide chain consisting of a linear chain of many beta 1-4 D-glucose and having the H-bonding helps in providing the good stability and mechanical strength.
Complete answer:
Cellulose has a composition of 4.44 % carbon 6.17 % hydrogen and 49.39% of oxygen. We chemically represent cellulose as \[(C6H10O5)n\]. (n) representing the degree of polymerization and the amount of glucose groups present in cellulose. Due to the strong hydrogen bonding between the cellulose molecule, cellulose provides high strength and stability to the plant. The structure of cellulose resembles that of starch which contains a minimum of 500 glucose molecules which makes it a polysaccharide and presence of hydrogen bonds make it very strongly bonded.
Cellulose is a very essential structure of cell walls in green plants and may be in some algae etc. Cellulose is present in abundance in Earth’s surface as an organic polymer cellulose is also used in the production of paper and paperboard. When the cellulose is present in smaller quantities it can be used to derive the product of rayon and cellophane.
The cellulose can be converted from energy crops to biofuels which is known as cellulosic ethanol and is a part of renewable sources of energy. While using the cellulose in industries we extract the cellulose from cotton or wood pulp and cellulose is also found in the ruminants for termites which can digest the cellulose by any symbiotic relationship.
In humans cellulose cannot be digested it is an insoluble dietary component and comes out directly in the faeces. Cellulose does not consist of any taste and smell for its hydrophilic in nature and it is not soluble in water.
The correct option is (C).
Note : Cellulose can be condensed through the glycosidic bond. In cellulose there is no branching or coiling of the molecule because it is a straight chain compound cellulose often provides high tensile strength to the plants and their stems and it is further distributed into the matrix of Lignin.
Complete answer:
Cellulose has a composition of 4.44 % carbon 6.17 % hydrogen and 49.39% of oxygen. We chemically represent cellulose as \[(C6H10O5)n\]. (n) representing the degree of polymerization and the amount of glucose groups present in cellulose. Due to the strong hydrogen bonding between the cellulose molecule, cellulose provides high strength and stability to the plant. The structure of cellulose resembles that of starch which contains a minimum of 500 glucose molecules which makes it a polysaccharide and presence of hydrogen bonds make it very strongly bonded.
Cellulose is a very essential structure of cell walls in green plants and may be in some algae etc. Cellulose is present in abundance in Earth’s surface as an organic polymer cellulose is also used in the production of paper and paperboard. When the cellulose is present in smaller quantities it can be used to derive the product of rayon and cellophane.

The cellulose can be converted from energy crops to biofuels which is known as cellulosic ethanol and is a part of renewable sources of energy. While using the cellulose in industries we extract the cellulose from cotton or wood pulp and cellulose is also found in the ruminants for termites which can digest the cellulose by any symbiotic relationship.
In humans cellulose cannot be digested it is an insoluble dietary component and comes out directly in the faeces. Cellulose does not consist of any taste and smell for its hydrophilic in nature and it is not soluble in water.
The correct option is (C).
Note : Cellulose can be condensed through the glycosidic bond. In cellulose there is no branching or coiling of the molecule because it is a straight chain compound cellulose often provides high tensile strength to the plants and their stems and it is further distributed into the matrix of Lignin.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




