
CFCs stands for:
(A) Carbon Fluorine Compounds
(B) Carbon Fluoro Compounds
(C) Chloro Fluoro Carbon
(D) Chlorine Fluoro Compounds
Answer
585.3k+ views
Hint: CFCs are gases. These are the form of hydrocarbons and its derivatives. These are partly or fully hydrogenated paraffin hydrocarbons. It is a volatile derivative of methane, propane and ethane.
Complete step by step solution:
CFCs are the derivative of carbon, chlorine, fluorine and hydrogen atoms. It is abbreviated as chlorofluorocarbon compounds. The structures of CFCs are tetrahedral in geometry like that of methane molecules. Due to different size and nuclear charge of different atoms, CFCs deviate from perfect tetrahedral geometry. The structures of CFCs are
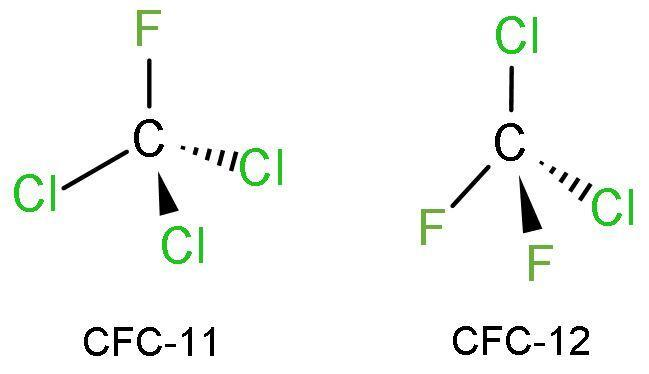
Due to the substitution of chlorine atoms in the compound’s structure, the densities of CFCs are higher than that of normal alkanes.
These compounds are chemically stable, non-flammable, odourless and tasteless. These compounds are highly volatile.
CFCs stands for Chloro Fluoro Carbon, which is option (C).
Additional information:
Applications of CFCs are:
(1) This gas is used in various purposes like refrigerants, solvents and aerosol sprays.
(2) Used as propellants in medicinal applications.
Note: This gas is very harmful to our environment and ecology. The harmful effects of CFCs include,
(1) Depletion of the ozone layer: Ozone layer is a layer which is present in the stratosphere to prevent entrance of harmful UV rays in the earth’s atmosphere. But CFCs create holes in the ozone layer which leads to the entrance of UV rays which causes skin cancer, etc.
(2) Even inhalation of CFCs gases affects the central nervous system. Other effects are tremors, headaches, and disturbs the heart rhythms.
Complete step by step solution:
CFCs are the derivative of carbon, chlorine, fluorine and hydrogen atoms. It is abbreviated as chlorofluorocarbon compounds. The structures of CFCs are tetrahedral in geometry like that of methane molecules. Due to different size and nuclear charge of different atoms, CFCs deviate from perfect tetrahedral geometry. The structures of CFCs are

Due to the substitution of chlorine atoms in the compound’s structure, the densities of CFCs are higher than that of normal alkanes.
These compounds are chemically stable, non-flammable, odourless and tasteless. These compounds are highly volatile.
CFCs stands for Chloro Fluoro Carbon, which is option (C).
Additional information:
Applications of CFCs are:
(1) This gas is used in various purposes like refrigerants, solvents and aerosol sprays.
(2) Used as propellants in medicinal applications.
Note: This gas is very harmful to our environment and ecology. The harmful effects of CFCs include,
(1) Depletion of the ozone layer: Ozone layer is a layer which is present in the stratosphere to prevent entrance of harmful UV rays in the earth’s atmosphere. But CFCs create holes in the ozone layer which leads to the entrance of UV rays which causes skin cancer, etc.
(2) Even inhalation of CFCs gases affects the central nervous system. Other effects are tremors, headaches, and disturbs the heart rhythms.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




