
$CHC{l_3}$on reaction with acetone in basic medium gives a compound used as:
(A) tear gas
(B) hypnotic
(C) pesticide
(D) anesthetic
Answer
582.9k+ views
Hint: Acetone contains carbonyl group in it. Carbonyl groups undergo addition reactions. This reaction based on the concept that chloroform $[CHC{l_3}]$ added on carbonyl group
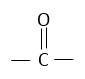 of acetone.
of acetone.
Complete step by step answer:
Let us write chemical equations for a given statement.
Chloroform $[CHC{l_3}]$ reacts with acetone
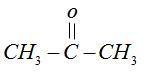 in basic medium $[KOH]$ and undergoes an addition reactions.
in basic medium $[KOH]$ and undergoes an addition reactions.
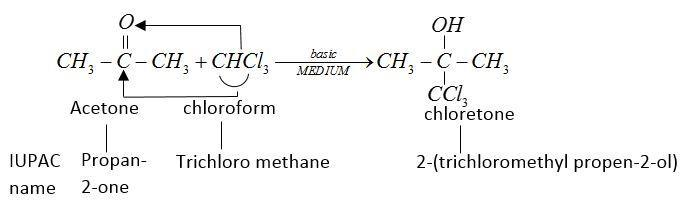
This chlorine formed is a sleep inducing drug.
Therefore, used as hypnotic.
Mechanism:
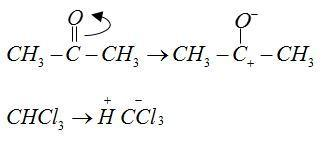
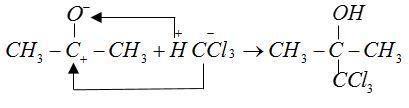
The anions of reactant $C{H_3}C{O^ - }$ and $CCl_3^ - $ form bonds with cations ${H^ + }$and $CH_3^ + $ respectively.
Therefore, from the above explanation the correct option is (B) Hypnotic.
Additional Information:
When acetone and chloroform are mixed together heat is evolved due to formation of hydrogen bonds between chloroform and acetone. This mixture forms a non-ideal solution and shows negative deviation from Raoult’s law.
But in the presence of strong alkali it gives chlorine.
due to increased intermolecular attraction and decreases vapor pressure.
At a specific composition, acetone and chloroform will form maximum boiling azeotrope.
Azeotrope are composed of one or more liquids having very similar boiling points even having different molecules weight.
Note:
For formation of chloretone basic medium is required mixture due to hydrogen bonding present between them.
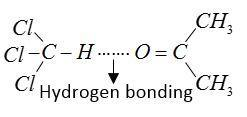

Complete step by step answer:
Let us write chemical equations for a given statement.
Chloroform $[CHC{l_3}]$ reacts with acetone
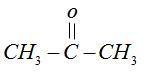

This chlorine formed is a sleep inducing drug.
Therefore, used as hypnotic.
Mechanism:
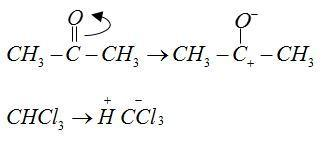
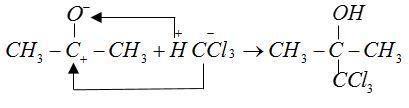
The anions of reactant $C{H_3}C{O^ - }$ and $CCl_3^ - $ form bonds with cations ${H^ + }$and $CH_3^ + $ respectively.
Therefore, from the above explanation the correct option is (B) Hypnotic.
Additional Information:
When acetone and chloroform are mixed together heat is evolved due to formation of hydrogen bonds between chloroform and acetone. This mixture forms a non-ideal solution and shows negative deviation from Raoult’s law.
But in the presence of strong alkali it gives chlorine.
due to increased intermolecular attraction and decreases vapor pressure.
At a specific composition, acetone and chloroform will form maximum boiling azeotrope.
Azeotrope are composed of one or more liquids having very similar boiling points even having different molecules weight.
Note:
For formation of chloretone basic medium is required mixture due to hydrogen bonding present between them.
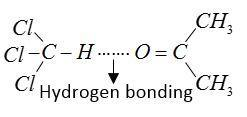
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




