
Chlorobenzene is extremely less reactive towards a nucleophilic substitution reaction. Give reasons for the same.
Answer
577.5k+ views
Hint: We know that in a nucleophilic substitution reaction, a nucleophile attacks the positively charged atom, where nucleophile is an electron-rich species. Some examples of nucleophiles are hydroxide ion, cyanide ion, etc.
Complete answer:
-The phenomenon in which delocalization of electrons causing the stabilization of molecules is known as
resonance.
-First, we will draw the resonance in chlorobenzene. The lone pair of chlorine atoms are delocalized in the benzene ring as-
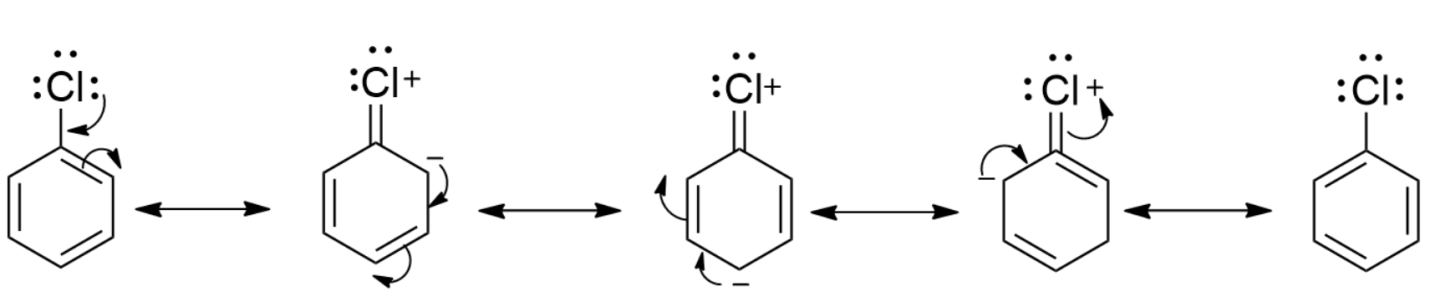
-From the resonance structures of chlorobenzene, we see that the lone pair of chlorine electrons are delocalized in the benzene ring and four resonating structures are formed. This causes the stabilization of the molecule. So, the activation energy for the displacement of halogen from benzene is very much higher than the displacement of alkyl halide.
-In chlorobenzene, the halogen atom (Cl) is bonded to a highly electronegative $s{{p}^{2}}$hybridized carbon atom of the benzene ring. So, nucleophilic substitution in chlorobenzene is not possible.
-Hence, the reasons behind less reactivity of chlorobenzene towards nucleophilic substitution reactions are resonance in chlorobenzene and $s{{p}^{2}}$ hybridized nature of carbon atoms bonded to the chlorine atom
Additional information:
In a chemical reaction, replacement of one group by another is known as substitution reaction. Substitution reactions are of three types, viz. electrophilic, nucleophilic and radical substitution reaction. An electrophile is a chemical species possessing electron-deficient nature and a nucleophile is a species possessing electron-rich nature.
Note:
You may get confused between nucleophilic and electrophilic substitution reaction. In electrophilic substitution, an electrophile generally displaces hydrogen atom from a compound and in nucleophilic substitution, nucleophile attacks positively charged carbon.
Complete answer:
-The phenomenon in which delocalization of electrons causing the stabilization of molecules is known as
resonance.
-First, we will draw the resonance in chlorobenzene. The lone pair of chlorine atoms are delocalized in the benzene ring as-

-From the resonance structures of chlorobenzene, we see that the lone pair of chlorine electrons are delocalized in the benzene ring and four resonating structures are formed. This causes the stabilization of the molecule. So, the activation energy for the displacement of halogen from benzene is very much higher than the displacement of alkyl halide.
-In chlorobenzene, the halogen atom (Cl) is bonded to a highly electronegative $s{{p}^{2}}$hybridized carbon atom of the benzene ring. So, nucleophilic substitution in chlorobenzene is not possible.
-Hence, the reasons behind less reactivity of chlorobenzene towards nucleophilic substitution reactions are resonance in chlorobenzene and $s{{p}^{2}}$ hybridized nature of carbon atoms bonded to the chlorine atom
Additional information:
In a chemical reaction, replacement of one group by another is known as substitution reaction. Substitution reactions are of three types, viz. electrophilic, nucleophilic and radical substitution reaction. An electrophile is a chemical species possessing electron-deficient nature and a nucleophile is a species possessing electron-rich nature.
Note:
You may get confused between nucleophilic and electrophilic substitution reaction. In electrophilic substitution, an electrophile generally displaces hydrogen atom from a compound and in nucleophilic substitution, nucleophile attacks positively charged carbon.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




