
Chloroethane is reacted with alcoholic potassium hydroxide. The product formed is:
A. ${C_2}{H_6}O$
B. ${C_2}{H_6}$
C. ${C_2}{H_4}$
D. ${C_2}{H_4}O$
Answer
569.4k+ views
Hint: In the given reaction elimination of the leaving group is taking place to form a side product which is a salt and the main product formed is an unsaturated hydrocarbon. Here, the alcoholic potassium hydroxide acts as a base.
Complete step by step answer:
The reaction of chloroethane with potassium hydroxide is shown below.
${C_2}{H_5}Cl\xrightarrow{{alc.KOH}}{C_2}{H_4} + HCl$
In this reaction chloroethane reacts with alcoholic potassium hydroxide to form ethene carrying a double bond and hydrochloric acid.
The mechanism is shown below.
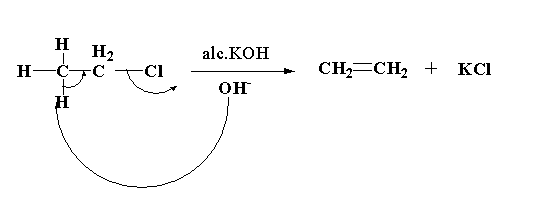
In this reaction, the alcoholic KOH acts as the base which will attack the hydrogen atom to form water. After removal of hydrogen the bond will shift to form ethene and remove the chlorine. The chlorine acts as a nucleophile which will attack the positive potassium ion to form potassium chloride.
Alcoholic potassium hydroxide is formed by dissolving anhydrous potassium hydroxide in ethanol. It is used in the elimination reactions which are water sensitive.
This reaction is an example of elimination reaction. The elimination reaction is used to convert a compound with saturated carbon (single bond) into unsaturated carbon (double bond).
In this reaction, the leaving group from the reactant is removed to form an unsaturated carbon compound. So, the correct answer is “Option C”.
Note:
This reaction is also known as dehydrohalogenation. Make sure that the potassium hydroxide used in the given reaction is alcoholic in nature. If aqueous potassium hydroxide is reacted with chloroethane, the main product which will be formed is alcohol with the removal of potassium chloride.
The reaction of chloroalkane with aqueous potassium hydroxide is shown below.
$RCl\xrightarrow{{aq.KOH}}ROH + KCl$
Where, R is an alkyl group.
Complete step by step answer:
The reaction of chloroethane with potassium hydroxide is shown below.
${C_2}{H_5}Cl\xrightarrow{{alc.KOH}}{C_2}{H_4} + HCl$
In this reaction chloroethane reacts with alcoholic potassium hydroxide to form ethene carrying a double bond and hydrochloric acid.
The mechanism is shown below.

In this reaction, the alcoholic KOH acts as the base which will attack the hydrogen atom to form water. After removal of hydrogen the bond will shift to form ethene and remove the chlorine. The chlorine acts as a nucleophile which will attack the positive potassium ion to form potassium chloride.
Alcoholic potassium hydroxide is formed by dissolving anhydrous potassium hydroxide in ethanol. It is used in the elimination reactions which are water sensitive.
This reaction is an example of elimination reaction. The elimination reaction is used to convert a compound with saturated carbon (single bond) into unsaturated carbon (double bond).
In this reaction, the leaving group from the reactant is removed to form an unsaturated carbon compound. So, the correct answer is “Option C”.
Note:
This reaction is also known as dehydrohalogenation. Make sure that the potassium hydroxide used in the given reaction is alcoholic in nature. If aqueous potassium hydroxide is reacted with chloroethane, the main product which will be formed is alcohol with the removal of potassium chloride.
The reaction of chloroalkane with aqueous potassium hydroxide is shown below.
$RCl\xrightarrow{{aq.KOH}}ROH + KCl$
Where, R is an alkyl group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




