
Chloroplantinic acid is:
A. Monobasic
B. Dibasic
C. Tribasic
D. Tetrabasic
Answer
578.1k+ views
Hint: Chloroplatinic acid is a reddish-brown color inorganic compound and soluble in water and forms mild acidic solution. The molecular formula of Chloroplatinic acid is ${{H}_{2}}PtC{{l}_{6}}$ with a molar mass of 409.81.
Complete step by step solution:
- In the question it is asked to find Chloroplantinic acid is a monobasic or dibasic or tribasic.
- To know about the acidity of the Chloroplatinic acid, we have to draw the structure of it first.
- The structure of Chloroplatinic acid is as follows.
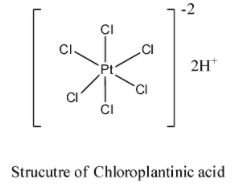
- Chloroplatinic acid is a coordination compound having an inner sphere and outer sphere.
- In the inner sphere all chlorine atoms are attached to platinum directly and the two hydrogen atoms are present in the outer sphere of the coordination complex.
- Means by dissolving the Chloroplatinic acid in water it will release two protons into the water.
- If any compound releases two protons into the solution then it is called dibasic compound.
- Therefore Chloroplatinic acid is a dibasic compound.
So, the correct option is B.
Additional information:
- Chloroplatinic acid is also called as Hexachloroplatinic acid due to the presence of sic chlorine atoms.
- Chloroplatinic acid is going to form by the addition of platinum in aqua regia.
Note: Platinum is very stable metal and wont react with other chemicals that much easily. To prepare Chloroplantinic acid platinum is added to a highly acidic solution called aqua regia. Aqua regia is a mixture of nitric acid and hydrochloric acid.
Complete step by step solution:
- In the question it is asked to find Chloroplantinic acid is a monobasic or dibasic or tribasic.
- To know about the acidity of the Chloroplatinic acid, we have to draw the structure of it first.
- The structure of Chloroplatinic acid is as follows.

- Chloroplatinic acid is a coordination compound having an inner sphere and outer sphere.
- In the inner sphere all chlorine atoms are attached to platinum directly and the two hydrogen atoms are present in the outer sphere of the coordination complex.
- Means by dissolving the Chloroplatinic acid in water it will release two protons into the water.
- If any compound releases two protons into the solution then it is called dibasic compound.
- Therefore Chloroplatinic acid is a dibasic compound.
So, the correct option is B.
Additional information:
- Chloroplatinic acid is also called as Hexachloroplatinic acid due to the presence of sic chlorine atoms.
- Chloroplatinic acid is going to form by the addition of platinum in aqua regia.
Note: Platinum is very stable metal and wont react with other chemicals that much easily. To prepare Chloroplantinic acid platinum is added to a highly acidic solution called aqua regia. Aqua regia is a mixture of nitric acid and hydrochloric acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




