
Choose the correct reason(s) for the stability of the lyophobic colloidal particles.
This question has multiple correct options
Answer
588.9k+ views
Hint: The colloidal particles which do not have any affinity towards the solvent are called lyophobic particles. It is not a reversible reaction unlike lyophilic particles which are reversible in nature. Lyophobic sols can be prepared by special methods only.
Complete step-by-step answer:
In chemistry, a colloid is a phase separated mixture in which one substance of dispersed particles either soluble or insoluble is suspended throughout another substance.
Unlike a solution, in which solute and solvent are present in one phase only, a colloid has two phases namely, dispersed phase and dispersion medium.
The dispersed phase particles have a diameter between approximately 1 and 1000 nanometers. Colloids may be translucent that show tyndall effect.
Lyophobic sols are the colloidal sols which show no affinity towards the dispersion medium. Lyophobic sols are generally considered more stable due to the following reasons:
- Preferential adsorption of ions on their surface from the solution
- The potential difference between the fixed layer and the diffused layer of opposite charges around the colloidal particles
Below is a diagram showing the stability of lyophobic sols.
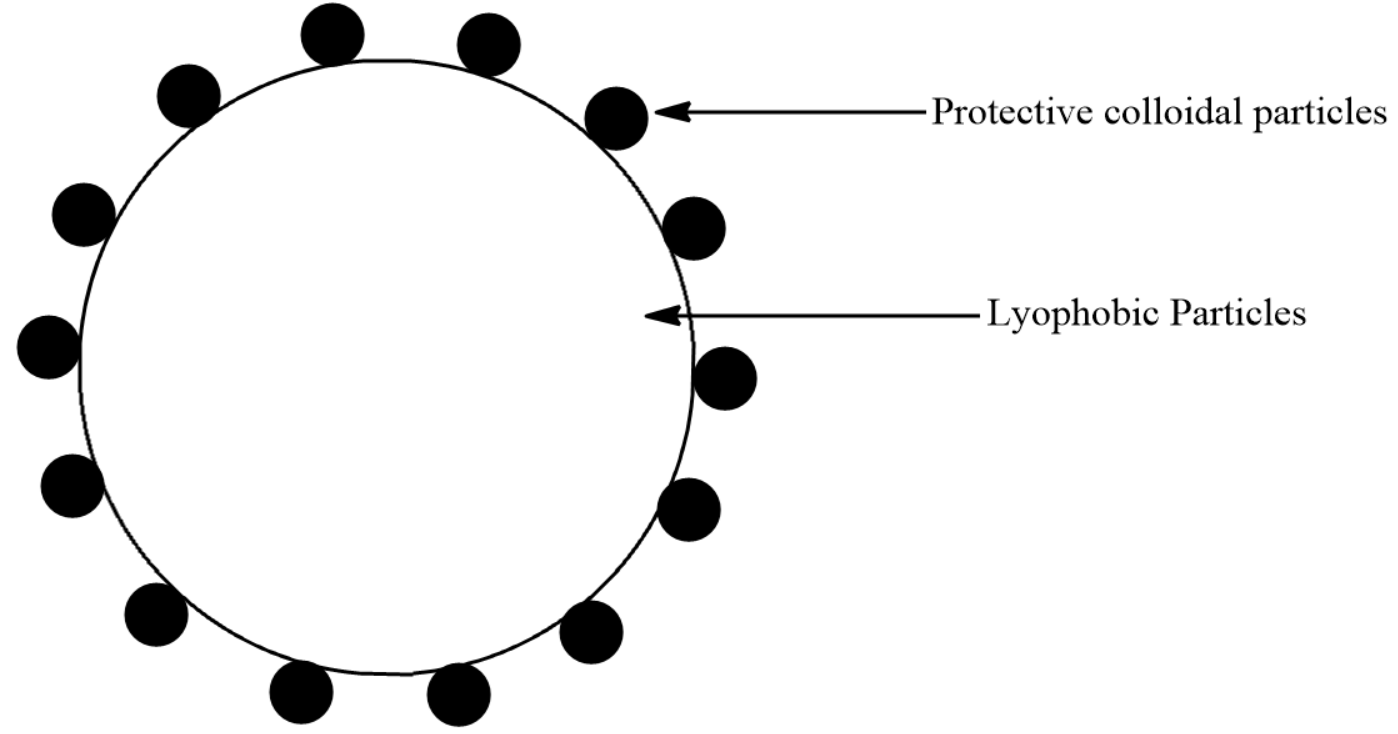
Therefore, the correct answer is options (A) and (D).
Note: Lyophobic sols unlike lyophilic sols cannot be prepared by directly mixing dispersed phase and dispersion medium. Additional stabilizers are added to form lyophobic sols. The particles of this sol can be detected only through an ultramicroscope.
Complete step-by-step answer:
In chemistry, a colloid is a phase separated mixture in which one substance of dispersed particles either soluble or insoluble is suspended throughout another substance.
Unlike a solution, in which solute and solvent are present in one phase only, a colloid has two phases namely, dispersed phase and dispersion medium.
The dispersed phase particles have a diameter between approximately 1 and 1000 nanometers. Colloids may be translucent that show tyndall effect.
Lyophobic sols are the colloidal sols which show no affinity towards the dispersion medium. Lyophobic sols are generally considered more stable due to the following reasons:
- Preferential adsorption of ions on their surface from the solution
- The potential difference between the fixed layer and the diffused layer of opposite charges around the colloidal particles
Below is a diagram showing the stability of lyophobic sols.

Therefore, the correct answer is options (A) and (D).
Note: Lyophobic sols unlike lyophilic sols cannot be prepared by directly mixing dispersed phase and dispersion medium. Additional stabilizers are added to form lyophobic sols. The particles of this sol can be detected only through an ultramicroscope.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




