
Choose the most appropriate answer for each of the following:
[Carbon tetrachloride, Hydrogen, Hydrogen chloride, Ammonium chloride]
Bonding in this molecule can be understood to involve coordinate bonding.
(A) Carbon tetrachloride
(B) Hydrogen
(C) Hydrogen chloride
(D) Ammonium chloride
Answer
591.6k+ views
Hint: Coordinate bond forms when one atom donates its pair of electrons to any other atom. So, only the atom that has a lone pair of electrons in its valence shell can donate to make this type of bond.
Complete step by step answer:
- Here, we are given to find which of the given molecules will have coordinate bonding.
- Coordinate bonds are also called covalent-coordinate bonds. Let’s analyze the bonding in all the given compounds.
i) $CC{{l}_{4}}$ (Carbon tetrachloride)
- Carbon is a tetravalent compound and it can form four covalent bonds. Thus, it can form one covalent bond each with four chlorine atoms. The structure of carbon tetrachloride is given as below.
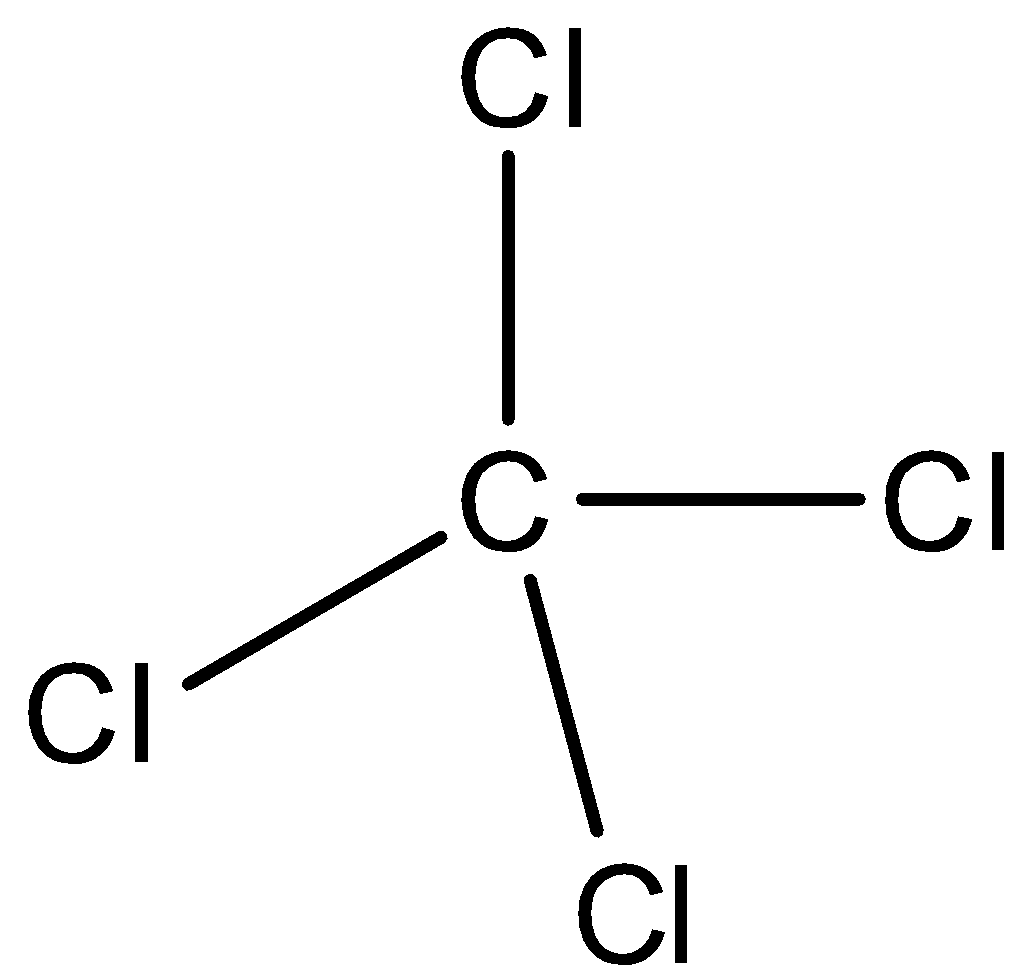
- Carbon atoms undergo $s{{p}^{3}}$ hybridization and form four hybrid orbitals involving one s-orbital and three p-orbitals. Each orbital has one electron each and shares one electron with chlorine atoms. Thus, all four bonds are formed by sharing of electrons. So, it involves only covalent bonds.
ii) ${{H}_{2}}$ (Hydrogen)
- Hydrogen has one electron in 1s orbital. So, it shares its electron with another hydrogen atom in order to complete the valence shell. Thus, there is a single bond between two hydrogen atoms in the structure. So, we can say that this bond is also a covalent bond.
iii) HCl (Hydrogen chloride)
- Hydrogen is a highly electropositive element and chlorine has high electronegativity. So, there will be a large difference for the affinity of electrons between them. So, it will result in an ionic bond between them. Hydrogen ions will lose one electron and chlorine atoms will accept that electron and hence ionic bonds will be formed. It can be shown as below.
\[{{H}^{+}}C{{l}^{-}}\]
iv) $N{{H}_{4}}Cl$ (Ammonium chloride)
- This compound involves coordinate bonding. Let’s see its structure.
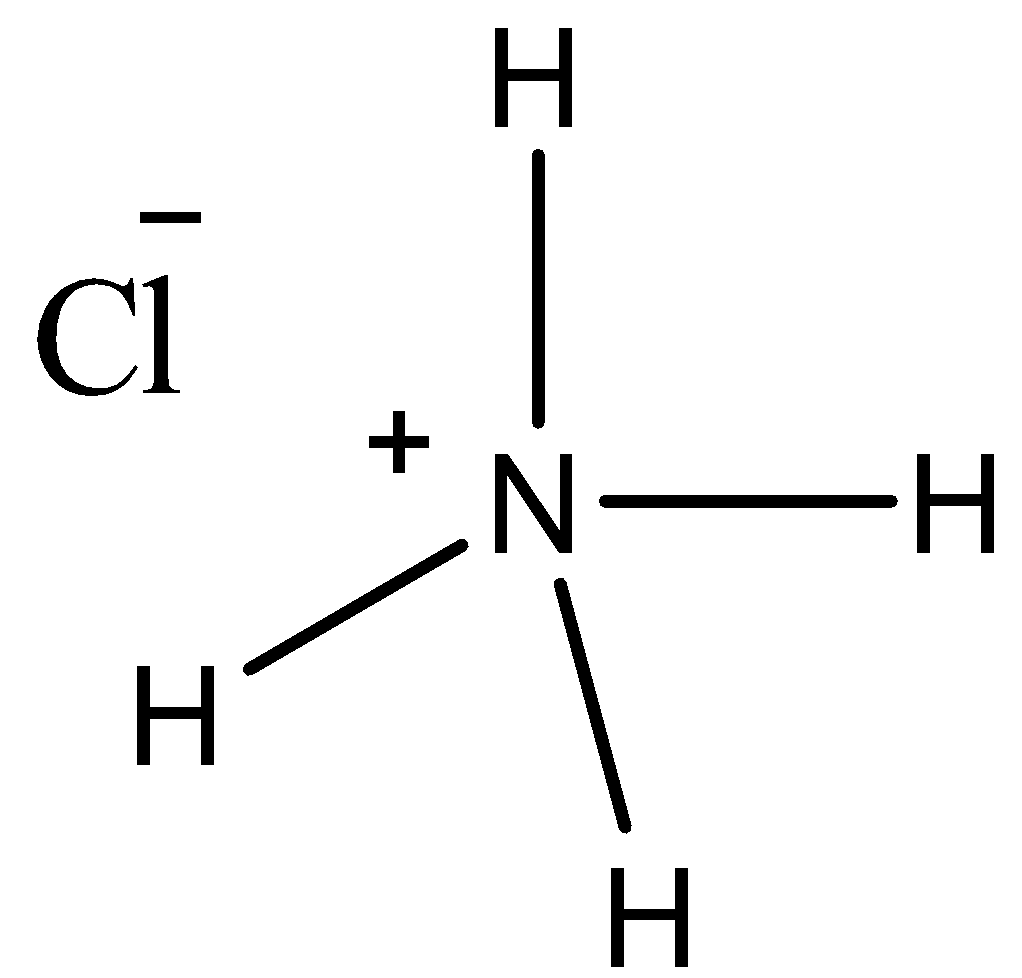
- We know that nitrogen atoms can form three covalent bonds as it has three electrons in its valence orbital. Here, the nitrogen atom will donate the pair of electrons to hydrogen and there, it will form a coordinate bond. So, as a result nitrogen will have a positive charge on it. So, it will make an ionic bond with chloride ions. Thus, this compound involves one coordinate bond.
So, the correct answer is “Option D”.
Note: Do not get confused between covalent bonding and coordinate bonding. Covalent bond results from sharing of one electron each from both the atoms to form a bond. Coordinate bond require an electron pair donor which is called ligand.
Complete step by step answer:
- Here, we are given to find which of the given molecules will have coordinate bonding.
- Coordinate bonds are also called covalent-coordinate bonds. Let’s analyze the bonding in all the given compounds.
i) $CC{{l}_{4}}$ (Carbon tetrachloride)
- Carbon is a tetravalent compound and it can form four covalent bonds. Thus, it can form one covalent bond each with four chlorine atoms. The structure of carbon tetrachloride is given as below.

- Carbon atoms undergo $s{{p}^{3}}$ hybridization and form four hybrid orbitals involving one s-orbital and three p-orbitals. Each orbital has one electron each and shares one electron with chlorine atoms. Thus, all four bonds are formed by sharing of electrons. So, it involves only covalent bonds.
ii) ${{H}_{2}}$ (Hydrogen)
- Hydrogen has one electron in 1s orbital. So, it shares its electron with another hydrogen atom in order to complete the valence shell. Thus, there is a single bond between two hydrogen atoms in the structure. So, we can say that this bond is also a covalent bond.
iii) HCl (Hydrogen chloride)
- Hydrogen is a highly electropositive element and chlorine has high electronegativity. So, there will be a large difference for the affinity of electrons between them. So, it will result in an ionic bond between them. Hydrogen ions will lose one electron and chlorine atoms will accept that electron and hence ionic bonds will be formed. It can be shown as below.
\[{{H}^{+}}C{{l}^{-}}\]
iv) $N{{H}_{4}}Cl$ (Ammonium chloride)
- This compound involves coordinate bonding. Let’s see its structure.

- We know that nitrogen atoms can form three covalent bonds as it has three electrons in its valence orbital. Here, the nitrogen atom will donate the pair of electrons to hydrogen and there, it will form a coordinate bond. So, as a result nitrogen will have a positive charge on it. So, it will make an ionic bond with chloride ions. Thus, this compound involves one coordinate bond.
So, the correct answer is “Option D”.
Note: Do not get confused between covalent bonding and coordinate bonding. Covalent bond results from sharing of one electron each from both the atoms to form a bond. Coordinate bond require an electron pair donor which is called ligand.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




