
Cis-2-butene and trans-2-butene are:
A. Geometrical isomers
B. Diastereomers
C. Enantiomers
D. Position isomers
Answer
578.7k+ views
Hint: Isomers are compounds that possess the same molecular formula but differ from each other in physical and chemical properties. Since isomers have the same molecular formula the difference in their properties might be due to different modes of combination of arrangement of atoms within the molecule.
Complete answer:
The isomers which differ only in the orientation of atoms in space are known as stereoisomers and this phenomenon is known as stereoisomerism. One of the types is geometrical isomerism, isomers which possess the same molecular and structural formula but differ in arrangement of atoms or groups in space around the double bonds are known as geometrical isomers and the phenomenon is known as geometrical isomerism.
Such isomers are not superimposable, that is if you try to put one compound over another. You will find discrepancy and hence they cannot be superimposed on each other.
Since the above compounds have double bonds and are alkenes therefore are geometrical isomers. Cis-trans isomerism is shown when similar groups are on the same side it is cis and if same groups are on the opposite side it is trans isomerism. Let us draw the structure of both the above mentioned compounds to get a clearer overview.
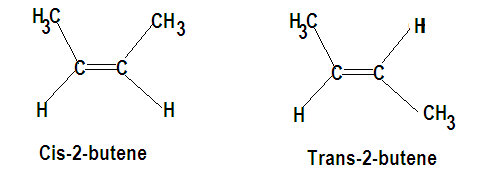
In other words, stereoisomerism is exhibited by such compounds which have identical molecular structure but different configurations. The other isomers are structural isomers.
Hence we can conclude that the above hydrocarbon compounds are geometrical isomers.
Therefore the correct option is A.
Note: Certain differentiating properties for cis-trans isomerism is dipole moment, melting point etc. as per stability is concerned, generally trans-isomer is more stable than cis-isomer as in cis form there will be steric repulsion.
Complete answer:
The isomers which differ only in the orientation of atoms in space are known as stereoisomers and this phenomenon is known as stereoisomerism. One of the types is geometrical isomerism, isomers which possess the same molecular and structural formula but differ in arrangement of atoms or groups in space around the double bonds are known as geometrical isomers and the phenomenon is known as geometrical isomerism.
Such isomers are not superimposable, that is if you try to put one compound over another. You will find discrepancy and hence they cannot be superimposed on each other.
Since the above compounds have double bonds and are alkenes therefore are geometrical isomers. Cis-trans isomerism is shown when similar groups are on the same side it is cis and if same groups are on the opposite side it is trans isomerism. Let us draw the structure of both the above mentioned compounds to get a clearer overview.

In other words, stereoisomerism is exhibited by such compounds which have identical molecular structure but different configurations. The other isomers are structural isomers.
Hence we can conclude that the above hydrocarbon compounds are geometrical isomers.
Therefore the correct option is A.
Note: Certain differentiating properties for cis-trans isomerism is dipole moment, melting point etc. as per stability is concerned, generally trans-isomer is more stable than cis-isomer as in cis form there will be steric repulsion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




