
When ${{C}}{{{O}}_{{2}}}$ is passed through sodium metaborate, which is found as a product?
A.Sodium borate
B.Borax
C.Boric acid
D.None of these
Answer
565.2k+ views
Hint: Sodium metaborate has high solubility. At high solubility, it can form higher concentrations of borate ions. Sodium tetraborate is also called borax, which is prepared from Sodium metaborate. Even the manufacturing of borosilicate glasses is done by the use of sodium metaborate. Borax can be fused with sodium hydroxide to produce sodium metaborate.
Complete step by step answer:
-When ${{C}}{{{O}}_{{2}}}$ is passed through sodium metaborate, we get sodium tetraborate and sodium carbonate as a product. As we already mentioned above sodium tetraborate is also called borax. Option (B) is right.
-Boric acid is used to prepare sodium metaborate. On hydrolyzing borax, we get boric acid and hydroxyl ions. Therefore sodium borate and boric acid are not the product when ${{C}}{{{O}}_{{2}}}$ is passed through sodium metaborate.
The chemical formula of sodium metaborate is ${{NaB}}{{{O}}_{{2}}}$
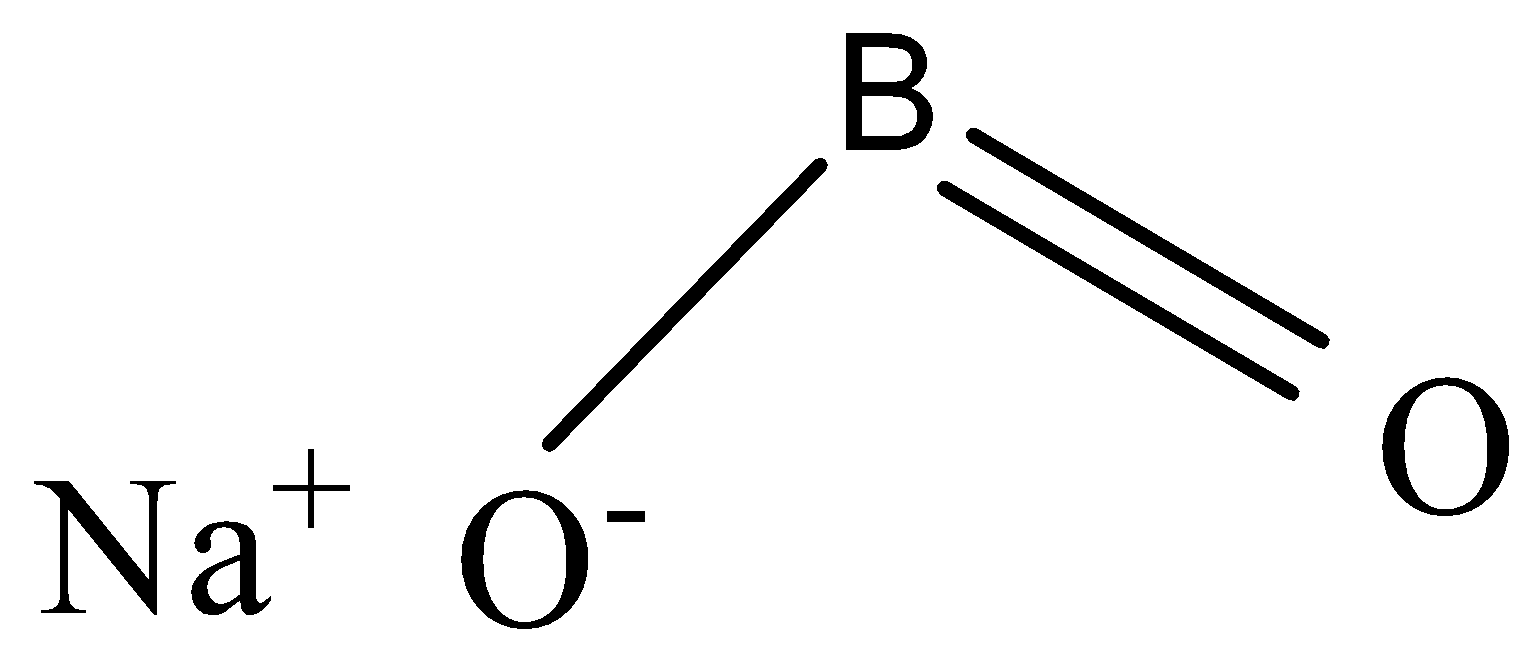
-To ${{NaB}}{{{O}}_{{2}}}$ , when ${{C}}{{{O}}_{{2}}}$ is passed, sodium tetraborate is formed, and in addition sodium carbonate is attained.
${{4NaB}}{{{O}}_{{2}}}{{ + C}}{{{O}}_{{2}}} \to {{N}}{{{a}}_{{2}}}{{C}}{{{O}}_{{3}}}{{ + N}}{{{a}}_{{2}}}{{{B}}_{{4}}}{{{O}}_{{7}}}$
Thus, the correct answer is an option (B).
Note:
When the borax formed is heated, it undergoes transitions. First, it loses water molecules and swells. Then it turns into a transparent liquid. It then solidifies to form a glass-like material called a borax bead. We know borax bead test which is used in qualitative analysis of certain coloured cations. Borax is heated in the loop of platinum wire and a colourless glassy bead is formed. When the coloured salt touches this bead, it gets a characteristic colour. This colour indicates the type of cations present. For example, the red colour of the bead shows copper ${{C}}{{{u}}^{{{2 + }}}}$ , pink shows manganese.
Complete step by step answer:
-When ${{C}}{{{O}}_{{2}}}$ is passed through sodium metaborate, we get sodium tetraborate and sodium carbonate as a product. As we already mentioned above sodium tetraborate is also called borax. Option (B) is right.
-Boric acid is used to prepare sodium metaborate. On hydrolyzing borax, we get boric acid and hydroxyl ions. Therefore sodium borate and boric acid are not the product when ${{C}}{{{O}}_{{2}}}$ is passed through sodium metaborate.
The chemical formula of sodium metaborate is ${{NaB}}{{{O}}_{{2}}}$

-To ${{NaB}}{{{O}}_{{2}}}$ , when ${{C}}{{{O}}_{{2}}}$ is passed, sodium tetraborate is formed, and in addition sodium carbonate is attained.
${{4NaB}}{{{O}}_{{2}}}{{ + C}}{{{O}}_{{2}}} \to {{N}}{{{a}}_{{2}}}{{C}}{{{O}}_{{3}}}{{ + N}}{{{a}}_{{2}}}{{{B}}_{{4}}}{{{O}}_{{7}}}$
Thus, the correct answer is an option (B).
Note:
When the borax formed is heated, it undergoes transitions. First, it loses water molecules and swells. Then it turns into a transparent liquid. It then solidifies to form a glass-like material called a borax bead. We know borax bead test which is used in qualitative analysis of certain coloured cations. Borax is heated in the loop of platinum wire and a colourless glassy bead is formed. When the coloured salt touches this bead, it gets a characteristic colour. This colour indicates the type of cations present. For example, the red colour of the bead shows copper ${{C}}{{{u}}^{{{2 + }}}}$ , pink shows manganese.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




