
Column chromatography involves separation of a mixture over a column of adsorbent (stationary phase) packed in a glass tube. Depending upon the degree of adsorption complete separation takes place. In the given column, three colored bands x, y, z are formed. Identify the correct statement.
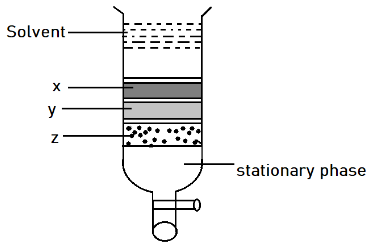
A) x, y and z are adsorbent to the same extent.
B) The most readily adsorbed component is retained near the top (x)
C) The most readily adsorbed component comes down (z)
D) x, y, z layers are formed according to the wavelengths of colors not the basis of adsorption.

Answer
547.2k+ views
Hint:In column chromatography which is a very famous instrumental method to separate the constituents in a compound. In this method there is a binding between the adsorbent and adsorbate by which some constituents get separated first and some after it. Try to figure out which solute among x, y and z adsorbed first.
Complete step-by-step answer:In the above diagram, see that there is a column which is filled with silica gel or some component which is having good absorption property. Thus was the solvent added, there are some interactions which act between different substances. So according to the figure it was seen that (x) get adsorbed first forming dark grey color band after it (y) get adsorbed and form light grey color. At last the (z) component gets absorbed it means that there is least interaction between the adsorbent and adsorbate. While the interactions are most in case of (x). Thus x, y and z do not absorb at the same extent.
Option B is correct.
Note: During the solvent addition from above of column, it was seen that the most intense interaction between solute and solvent gets adsorbed first and then one component gets separated. After that the second which has the most intense interaction or which is able to bind most strongly will get separate and after that we get the second components.
Complete step-by-step answer:In the above diagram, see that there is a column which is filled with silica gel or some component which is having good absorption property. Thus was the solvent added, there are some interactions which act between different substances. So according to the figure it was seen that (x) get adsorbed first forming dark grey color band after it (y) get adsorbed and form light grey color. At last the (z) component gets absorbed it means that there is least interaction between the adsorbent and adsorbate. While the interactions are most in case of (x). Thus x, y and z do not absorb at the same extent.
Option B is correct.
Note: During the solvent addition from above of column, it was seen that the most intense interaction between solute and solvent gets adsorbed first and then one component gets separated. After that the second which has the most intense interaction or which is able to bind most strongly will get separate and after that we get the second components.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




