
Complete the following reaction equation :
A. \[{C_6}{H_5}{N_2}Cl + {H_3}P{O_2} + {H_2}O \to \]
B. ${C_6}{H_5}N{H_2} + B{r_2}\left( {aq.} \right) \to $
Answer
582.9k+ views
Hint: ${C_6}{H_5}{N_2}Cl$ is known as benzenediazonium-chloride. It will undergo a substitution reaction while reacting with hypophosphorous acid .
Complete step by step answer: ${C_6}{H_5}{N_2}Cl$ is known as benzenediazonium chloride. It is a saet of a diazonium cation and chloride. It exists as a colourless solid.
As we know the diazonium group is a very good leaving group and being positively charged, it takes away the two electrons of the bond that it has with the phenyl group. So, the incoming group that replaces $N_2^ + $ has to be a nucleophile.
-Hence, from ${H_3}P{O_2}$ the nucleophilic group that can be formed will be ${H_3}PO_2^ - $. The presence of ${H_2}O$ in the acid can also act as a nucleophile through its oxygen.
The products formed by the reaction of benzenediazonium chloride are ${C_6}{H_6},{N_2},HCl$ and ${H_3}P{O_3}$.
The reaction equation can be written as :-
${C_6}{H_5}{N_2}Cl + {H_3}P{O_2} + {H_2}O \to {C_6}{H_6} + {N_2} + {H_3}P{O_3} + HCl$
-when ${C_6}{H_5}{N_2}Cl$ that is benzenediazonium chloride reacts with $B{r_2}$ then the product will be $2,4,6 - tribromoaniline$ the reaction equation involved is :-
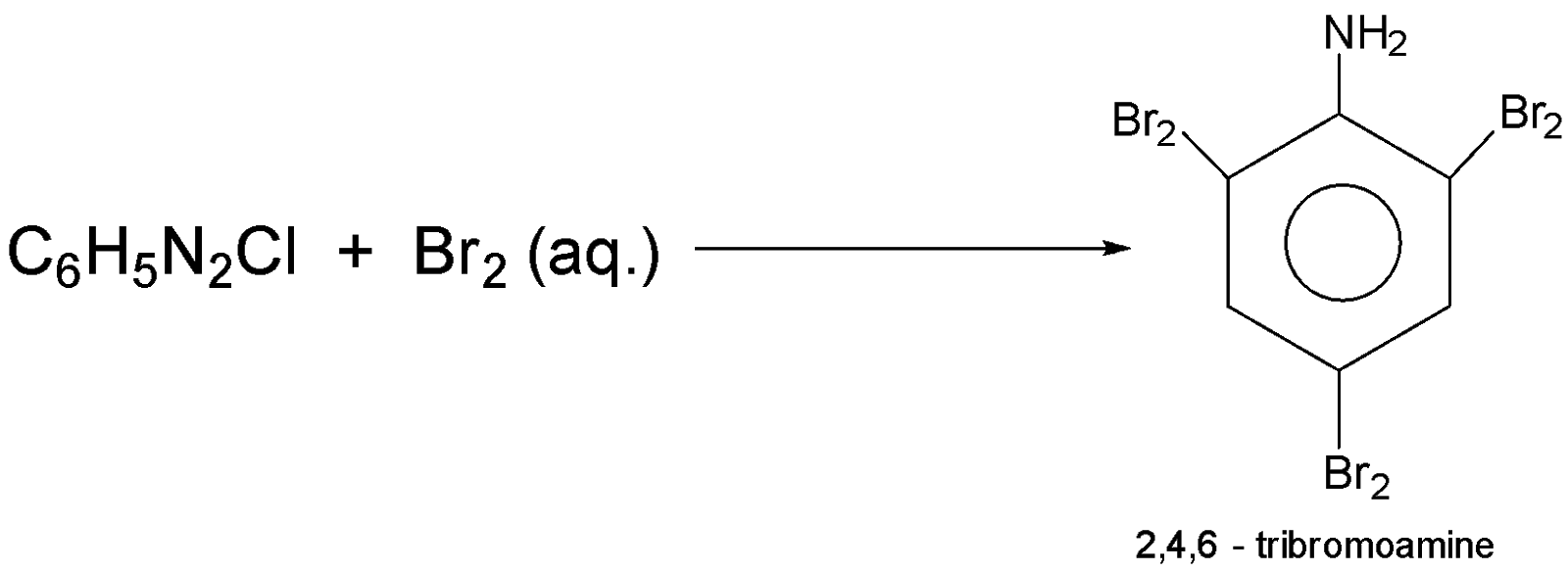
Additional Information: Benzenediazonium chloride is the parent member of the aryl diazonium compound that has use in organic chemistry; this salt is highly unstable.
Note: The diazo group $\left( {{N_2}} \right)$can be replaced with many other groups, usually anions, that give a variety of substituted phenyl derivatives.
Moreover, this compound seems to be explosive.
Complete step by step answer: ${C_6}{H_5}{N_2}Cl$ is known as benzenediazonium chloride. It is a saet of a diazonium cation and chloride. It exists as a colourless solid.
As we know the diazonium group is a very good leaving group and being positively charged, it takes away the two electrons of the bond that it has with the phenyl group. So, the incoming group that replaces $N_2^ + $ has to be a nucleophile.
-Hence, from ${H_3}P{O_2}$ the nucleophilic group that can be formed will be ${H_3}PO_2^ - $. The presence of ${H_2}O$ in the acid can also act as a nucleophile through its oxygen.
The products formed by the reaction of benzenediazonium chloride are ${C_6}{H_6},{N_2},HCl$ and ${H_3}P{O_3}$.
The reaction equation can be written as :-
${C_6}{H_5}{N_2}Cl + {H_3}P{O_2} + {H_2}O \to {C_6}{H_6} + {N_2} + {H_3}P{O_3} + HCl$
-when ${C_6}{H_5}{N_2}Cl$ that is benzenediazonium chloride reacts with $B{r_2}$ then the product will be $2,4,6 - tribromoaniline$ the reaction equation involved is :-
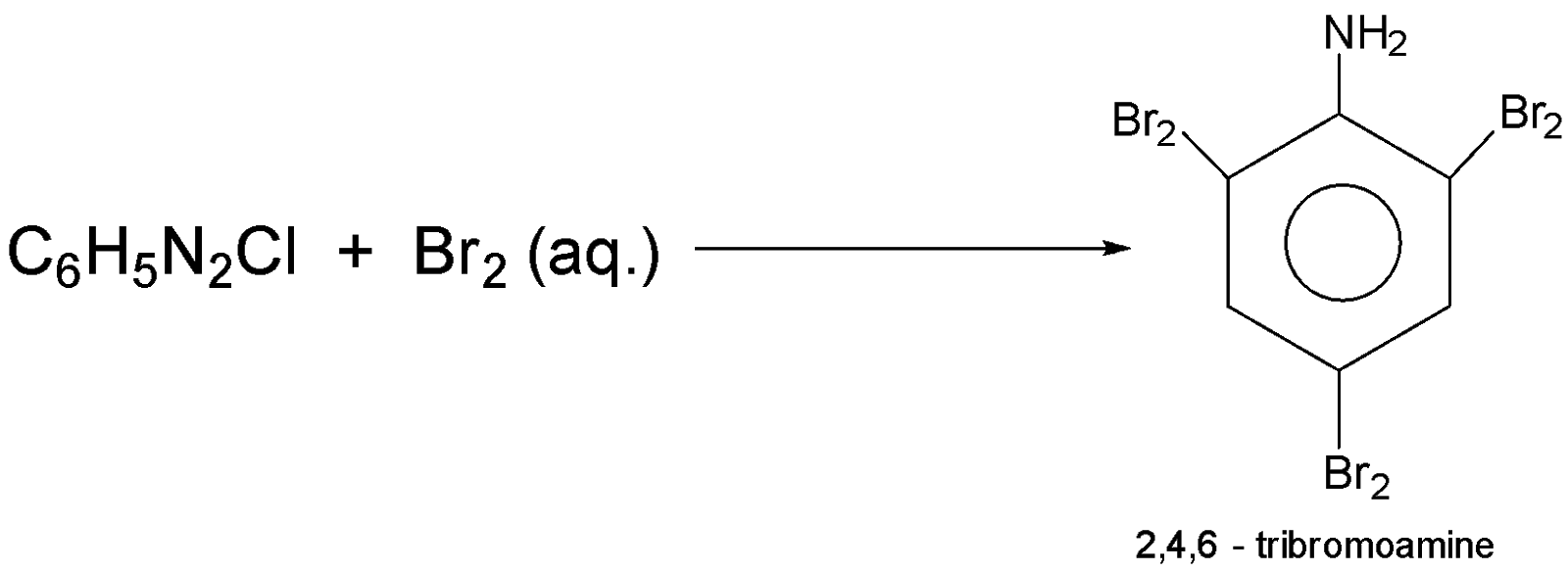
Additional Information: Benzenediazonium chloride is the parent member of the aryl diazonium compound that has use in organic chemistry; this salt is highly unstable.
Note: The diazo group $\left( {{N_2}} \right)$can be replaced with many other groups, usually anions, that give a variety of substituted phenyl derivatives.
Moreover, this compound seems to be explosive.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




