
Complete the following reactions:
a.
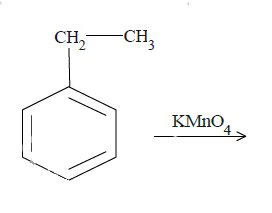
b.
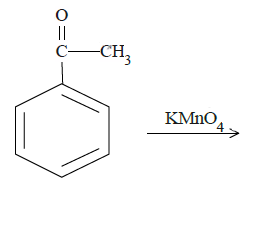
c.
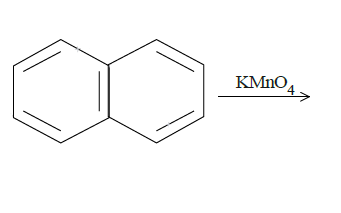
d.
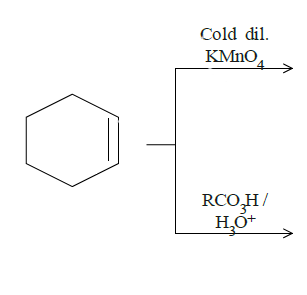




Answer
593.1k+ views
Hint: Reaction with $KMn{O_4}$ attacks the benzylic carbon which is the carbon atom next to benzene. If there is at least one hydrogen atom on the carbon atom, the reaction will cut the carbon atom from other links and convert it to a carboxyl group. The last problem is the epoxidation reaction where the double bond will break to form an epoxy.
Complete answer:
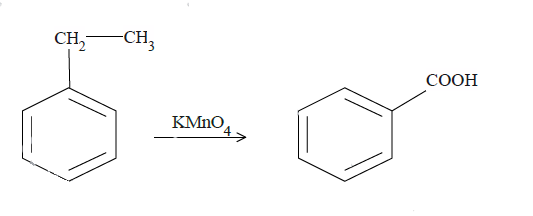
$KMn{O_4}$ is a strong oxidizing agent and is most commonly used in oxidation reactions of organic compounds. The final results are often carboxylic compounds. In (a) and (b), reaction with $KMn{O_4}$ will convert the benzylic carbon to carboxyl group. Now it is very important that there is at least one hydrogen atom at the benzylic carbon position. $KMn{O_4}$ attacks at the weak bonds of the compounds like the covalent double or triple bonds. In case of aromatic compounds, the C-H bond for carbon at the alpha position with respect to benzene molecules is a weak bond. In case of (a), it will react with the benzyl carbon cutting its bond with the methyl group later and convert it to the carboxyl group.
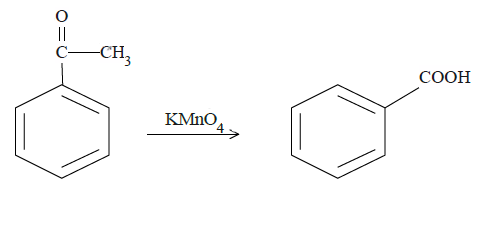
In case of (b), ketone carbon will be oxidized to the carboxyl group.
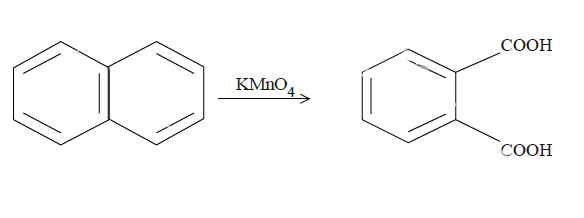
In (c), we have naphthalene reacted with $KMn{O_4}$. It will produce benzene with two carboxyl groups on adjacent carbon atoms on the benzene ring breaking one ring. The reaction is as follows:
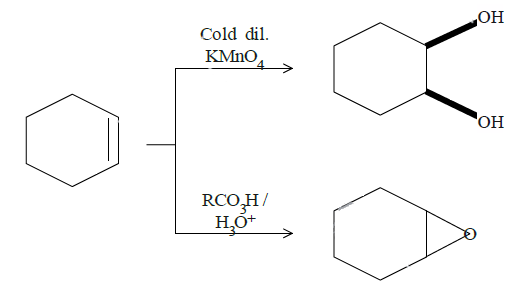
In question (d), we have been asked to give results for two reactions. In the top one, mild $KMn{O_4}$ will break the double bond and add hydroxyl groups to adjacent carbon atoms. The dark lines tell the bonds are on the same side of the original double bond since it is formed by breaking the double bond and attacking the adjacent carbon bonds from the same side by the manganate ion. The cold diluted conditions mean the conditions are mild and thus this product. Reaction on the bottom is an example of epoxidation. One oxygen from the reactant will attack the double bond and attach itself to both the carbon atoms. Both the reactions are as follows:
Note: In the first part of the last reaction, if there is heat added to the reaction and more concentrated $KMn{O_4}$ then the glycol we have estimated will oxidise further and also breaking the bond between the adjacent carbon atoms to form chain compound with carboxyl group at the ends. $KMn{O_4}$ is generally not used in the productions of aldehydes and ketones because of its strong oxidizing nature.
Complete answer:
$KMn{O_4}$ is a strong oxidizing agent and is most commonly used in oxidation reactions of organic compounds. The final results are often carboxylic compounds. In (a) and (b), reaction with $KMn{O_4}$ will convert the benzylic carbon to carboxyl group. Now it is very important that there is at least one hydrogen atom at the benzylic carbon position. $KMn{O_4}$ attacks at the weak bonds of the compounds like the covalent double or triple bonds. In case of aromatic compounds, the C-H bond for carbon at the alpha position with respect to benzene molecules is a weak bond. In case of (a), it will react with the benzyl carbon cutting its bond with the methyl group later and convert it to the carboxyl group.

In case of (b), ketone carbon will be oxidized to the carboxyl group.

In (c), we have naphthalene reacted with $KMn{O_4}$. It will produce benzene with two carboxyl groups on adjacent carbon atoms on the benzene ring breaking one ring. The reaction is as follows:

In question (d), we have been asked to give results for two reactions. In the top one, mild $KMn{O_4}$ will break the double bond and add hydroxyl groups to adjacent carbon atoms. The dark lines tell the bonds are on the same side of the original double bond since it is formed by breaking the double bond and attacking the adjacent carbon bonds from the same side by the manganate ion. The cold diluted conditions mean the conditions are mild and thus this product. Reaction on the bottom is an example of epoxidation. One oxygen from the reactant will attack the double bond and attach itself to both the carbon atoms. Both the reactions are as follows:

Note: In the first part of the last reaction, if there is heat added to the reaction and more concentrated $KMn{O_4}$ then the glycol we have estimated will oxidise further and also breaking the bond between the adjacent carbon atoms to form chain compound with carboxyl group at the ends. $KMn{O_4}$ is generally not used in the productions of aldehydes and ketones because of its strong oxidizing nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




