
Complex ${{[Fe{{L}_{6}}]}^{2+}}$ is yellow in color then expected magnetic moment (in BM) of complex will be (L $\to $ Monodentate neutral ligand) (Wavelength corresponding to pairing energy is 500 nm):
(a)- 0
(b)- $\sqrt{8}$
(c)- $\sqrt{15}$
(d)- $\sqrt{24}$
Answer
508.8k+ views
Hint: To find the magnetic moment we have to first, find the central metal atom in the complex and then write its electronic configuration in the ground state. Find the oxidation state of the central metal atom and write the configuration accordingly, and find the number of unpaired electrons. Use the formula $\sqrt{n(n+2)}$ to find the magnetic moment where n is the number of unpaired electrons.
Complete answer:
The given complex is:
${{[Fe{{L}_{6}}]}^{2+}}$
The central metal atom in this is iron, and the atomic number of iron is 26.
So, the electronic configuration of iron in the ground state will be:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{6}}$
Given that the L (ligand) is a monodentate neutral ligand, there will be no charge on it. As the overall charge on the complex is +2, so the sum of all the oxidation states of the elements in the complex will be equal to +2. The oxidation state of iron will be:
$x+6(0)=+2$
$x=+2$
So, the oxidation state of iron is +2. The electronic configuration of iron in +2 oxidation state will be:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{6}}$
So, the $3{{d}^{6}}$ will be represented as:
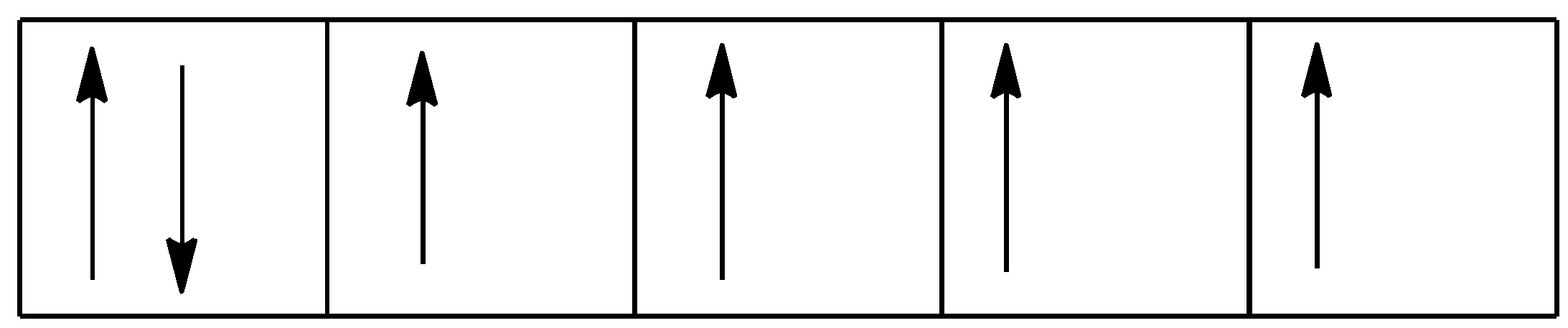
We can use the formula $\sqrt{n(n+2)}$ to find the magnetic moment where n is the number of unpaired electrons.
So, the numbers of unpaired electrons are 4. Putting this value in the formula, we get:
$\sqrt{n(n+2)}=\sqrt{4(4+2)}$
$Magnetic\text{ }moment=\sqrt{24}$
So, the correct answer is an option (d).
Note:
There will be no pairing in the complex because the energy at which the absorption occurs is 410 nm, and the minimum amount of wavelength required for the pairing is 500 nm.
Complete answer:
The given complex is:
${{[Fe{{L}_{6}}]}^{2+}}$
The central metal atom in this is iron, and the atomic number of iron is 26.
So, the electronic configuration of iron in the ground state will be:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{6}}$
Given that the L (ligand) is a monodentate neutral ligand, there will be no charge on it. As the overall charge on the complex is +2, so the sum of all the oxidation states of the elements in the complex will be equal to +2. The oxidation state of iron will be:
$x+6(0)=+2$
$x=+2$
So, the oxidation state of iron is +2. The electronic configuration of iron in +2 oxidation state will be:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}3{{d}^{6}}$
So, the $3{{d}^{6}}$ will be represented as:

We can use the formula $\sqrt{n(n+2)}$ to find the magnetic moment where n is the number of unpaired electrons.
So, the numbers of unpaired electrons are 4. Putting this value in the formula, we get:
$\sqrt{n(n+2)}=\sqrt{4(4+2)}$
$Magnetic\text{ }moment=\sqrt{24}$
So, the correct answer is an option (d).
Note:
There will be no pairing in the complex because the energy at which the absorption occurs is 410 nm, and the minimum amount of wavelength required for the pairing is 500 nm.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




