
Compound 'A' of molecular formula ${{C}_{4}}{{H}_{10}}O$ on treatment with Lucas reagent at room temperature gives compound 'B'. When compound 'B' is heated with alcoholic $KOH$, it gives isobutene. Compound 'A' and 'B' are respectively:
(A) 2-methyl-2-propanol and 2-methyl-2-chloropropane
(B) 2-methyl-1-propanol and 1-chloro-2-methylpropane
(C) 2-methyl-1-propanol and 2-methyl-2-chloropropane
(D) butan-2-ol and 2-chlorobutane
(E) none of the above
Answer
589.8k+ views
Hint: Write down the possible structures of organic compounds with molecular formula ${{C}_{4}}{{H}_{10}}O$. Now write the reaction of the possible compounds with Lucas reagent and note down the respective products that can possibly be compound 'B'. Now write the reaction of the products obtained from the previous reaction with alcoholic $KOH$ and see which compound gives isobutene as the product.
Complete step by step answer:
Lucas reagent is a solution of anhydrous zinc chloride and hydrochloric acid. In this reaction, chloride replaces the hydroxyl group changing the compound from alcohol to alkyl chloride.
When an alkyl chloride reacts with alcoholic KOH, it leads to the elimination of the chloride ion and a hydrogen atom from the adjacent carbon atom i.e. $\beta $ elimination.
We will try to write the chemical reaction of the above two reagents with the organic compounds given in the options.
Let us start with the first organic compound, the structure is given below:
2-methyl-2-propanol:
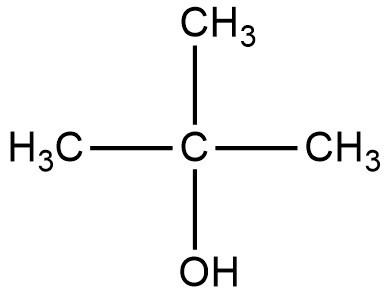
The reaction of Lucas reagent on 2-methyl-2-propanol gives 2-methyl-2-chloropropane.
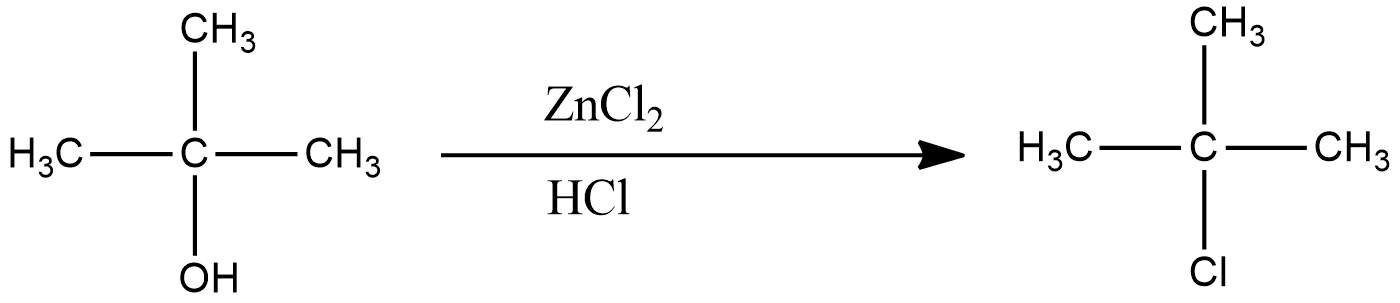
The reaction of 2-methyl-2-chloropropane gives 2-methylpropene.
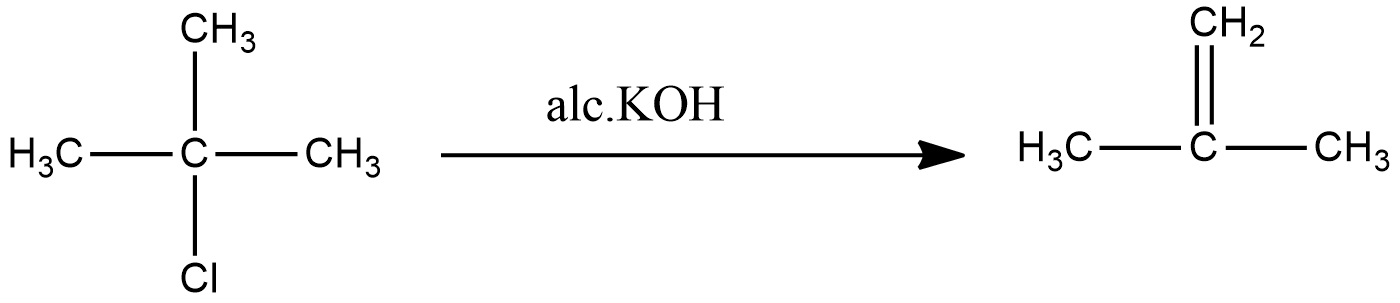
The common name for 2-methylpropene is isobutene. The final product matches with the final product mentioned in the question. Hence the compound (A) is 2-methyl-2-propanol and compound (B) is 2-methyl-2-chloropropane.
So, the correct answer is “Option A”.
Note: The Lucas test for alcohols is a test to differentiate between primary, secondary, and tertiary alcohols. It is based on the difference in reactivity of the three classes of alcohols with hydrogen halides through ${{S}_{N}}1$ reaction mechanism:
$\text{ROH + HCl }\to \text{ RCl + }{{\text{H}}_{2}}\text{O}$
The above reaction happens instantly for tertiary alcohols. This is confirmed by the solution turning turbid. Primary alcohols react on long withstanding.
Complete step by step answer:
Lucas reagent is a solution of anhydrous zinc chloride and hydrochloric acid. In this reaction, chloride replaces the hydroxyl group changing the compound from alcohol to alkyl chloride.
When an alkyl chloride reacts with alcoholic KOH, it leads to the elimination of the chloride ion and a hydrogen atom from the adjacent carbon atom i.e. $\beta $ elimination.
We will try to write the chemical reaction of the above two reagents with the organic compounds given in the options.
Let us start with the first organic compound, the structure is given below:
2-methyl-2-propanol:

The reaction of Lucas reagent on 2-methyl-2-propanol gives 2-methyl-2-chloropropane.
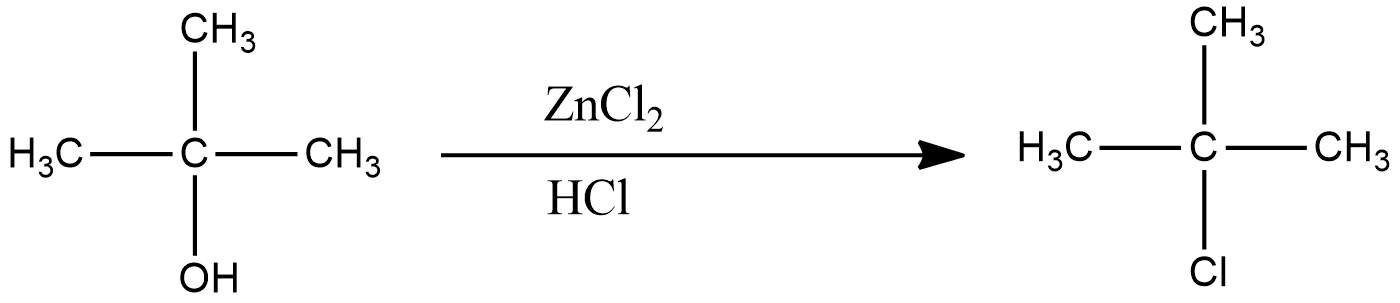
The reaction of 2-methyl-2-chloropropane gives 2-methylpropene.
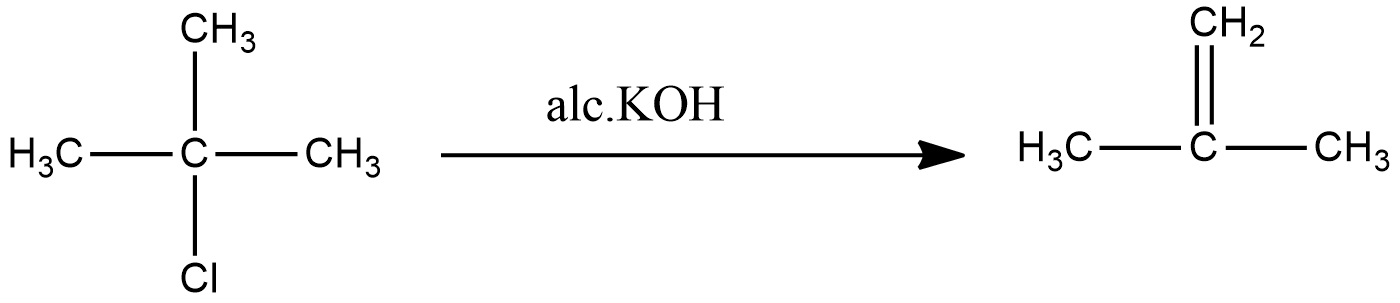
The common name for 2-methylpropene is isobutene. The final product matches with the final product mentioned in the question. Hence the compound (A) is 2-methyl-2-propanol and compound (B) is 2-methyl-2-chloropropane.
So, the correct answer is “Option A”.
Note: The Lucas test for alcohols is a test to differentiate between primary, secondary, and tertiary alcohols. It is based on the difference in reactivity of the three classes of alcohols with hydrogen halides through ${{S}_{N}}1$ reaction mechanism:
$\text{ROH + HCl }\to \text{ RCl + }{{\text{H}}_{2}}\text{O}$
The above reaction happens instantly for tertiary alcohols. This is confirmed by the solution turning turbid. Primary alcohols react on long withstanding.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




