
Compound C, has the molecular formula $ {C_7}{H_{12}} $ . On catalytic hydrogenation, one mole of C absorbs one mole of hydrogen gas and yields a compound with the molecular formula of $ {C_7}{H_{12}} $ . On ozonolysis and subsequent treatment with zinc and acetic acid, C yields only $ C{H_3}COC{H_2}C{H_2}C{H_2}C{H_2}C{H_2}CHO $ . The structure of C is:
(all diagrams are drawn using chemdraw)
(A)
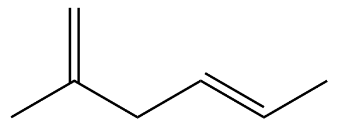
(B)
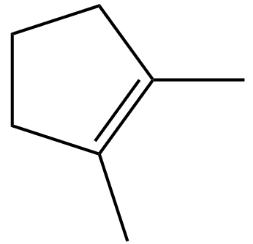
(C)
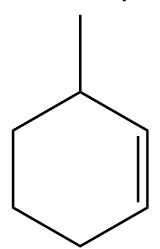
(D)
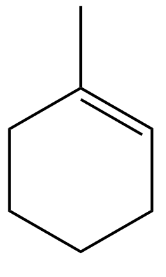




Answer
516.9k+ views
Hint : The ozonolysis reaction is the reaction in which the three atoms of oxygen attack a saturated and the unsaturated bonds present together to totally break and they have consequent oxygen attached to the. This reaction is usually used to break chains.
Complete Step By Step Answer:
Now we are given that only $ C{H_3}COC{H_2}C{H_2}C{H_2}C{H_2}C{H_2}CHO $ is the only compound to be formed out of the ozonolysis of the compound C.
For the existence of just one product after the ozonolysis and subsequent treatment with zinc and acetic acid the product C which goes under the above-mentioned reactions has to be a cyclic compound and also the hydrogenation of C Yields C with the same formula so, it confirms the cyclic nature of the compound.
Now we need to know what type of cyclic compound is it because on ozonolysis of the cyclic compound we get $ C{H_3}COC{H_2}C{H_2}C{H_2}C{H_2}C{H_2}CHO $ which has one keto compound and one aldehyde group. Now for the keto group to form we need the unsaturated carbon to be a third-degree carbon, which is the carbon is attached to three carbons other than hydrogen, while for the aldehyde group we need the carbon to be a second-degree carbon, that is the carbon is attached to two carbons other than the hydrogen.
Option A is discarded because of it being a straight chain.
Option B is discarded because the carbon across the double bond is both third degree.
Option C is discarded because both the unsaturated carbon in the ring is second degree carbon.
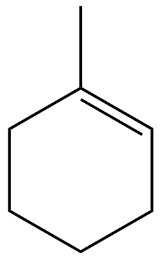
Option D is the correct option because one of the unsaturated carbons is second degree while the other is third degree which on ozonolysis gives $ C{H_3}COC{H_2}C{H_2}C{H_2}C{H_2}C{H_2}CHO $ .
Note :
Catalytic hydrogenation of a cyclic compound doesn’t take place for various reasons. One of the reasons that the reaction doesn’t take place is due to the geometry of the cyclic compound which doesn’t give the space to hydrogen to attack and also the cyclic double bonds are more stable and have more tension between the bonds.
Complete Step By Step Answer:
Now we are given that only $ C{H_3}COC{H_2}C{H_2}C{H_2}C{H_2}C{H_2}CHO $ is the only compound to be formed out of the ozonolysis of the compound C.
For the existence of just one product after the ozonolysis and subsequent treatment with zinc and acetic acid the product C which goes under the above-mentioned reactions has to be a cyclic compound and also the hydrogenation of C Yields C with the same formula so, it confirms the cyclic nature of the compound.
Now we need to know what type of cyclic compound is it because on ozonolysis of the cyclic compound we get $ C{H_3}COC{H_2}C{H_2}C{H_2}C{H_2}C{H_2}CHO $ which has one keto compound and one aldehyde group. Now for the keto group to form we need the unsaturated carbon to be a third-degree carbon, which is the carbon is attached to three carbons other than hydrogen, while for the aldehyde group we need the carbon to be a second-degree carbon, that is the carbon is attached to two carbons other than the hydrogen.
Option A is discarded because of it being a straight chain.
Option B is discarded because the carbon across the double bond is both third degree.
Option C is discarded because both the unsaturated carbon in the ring is second degree carbon.
Option D is the correct option because one of the unsaturated carbons is second degree while the other is third degree which on ozonolysis gives $ C{H_3}COC{H_2}C{H_2}C{H_2}C{H_2}C{H_2}CHO $ .

Note :
Catalytic hydrogenation of a cyclic compound doesn’t take place for various reasons. One of the reasons that the reaction doesn’t take place is due to the geometry of the cyclic compound which doesn’t give the space to hydrogen to attack and also the cyclic double bonds are more stable and have more tension between the bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




