
Consider the Argon atom. For how many electrons does this atom have ${{m}_{l}}=1$ ? [Atomic number of Ar = 18]
(a)- 1
(b)- 6
(c)- 4
(d)- 2
Answer
493.2k+ views
Hint: ${{m}_{l}}$ is the magnetic quantum number and l is the Azimuthal quantum number. With the help of l value we can find the magnetic quantum number. Given ${{m}_{l}}$=1, the values of the magnetic quantum number will range from -1 to 1.
Complete answer:
We know that there are four quantum numbers, i.e., principle quantum number, Azimuthal quantum number, magnetic quantum number, and spin quantum number.
${{m}_{l}}$ is the magnetic quantum number and l is the Azimuthal quantum number. With the help of l value we can find the magnetic quantum number. Given ${{m}_{l}}$=1, the values of the magnetic quantum number will range from -1 to 1.
The value of l is 1 when the subshell is p.
The given element in the question is Argon whose atomic number is 18 so, there are 18 electrons in argon atom. Its electronic configuration is given below:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}$
We know that there are three orbitals in the p-subshell, which are denoted as -1, 0, and 1. In argon there are 2p and 3p orbitals. The arrangement of electrons is given below:
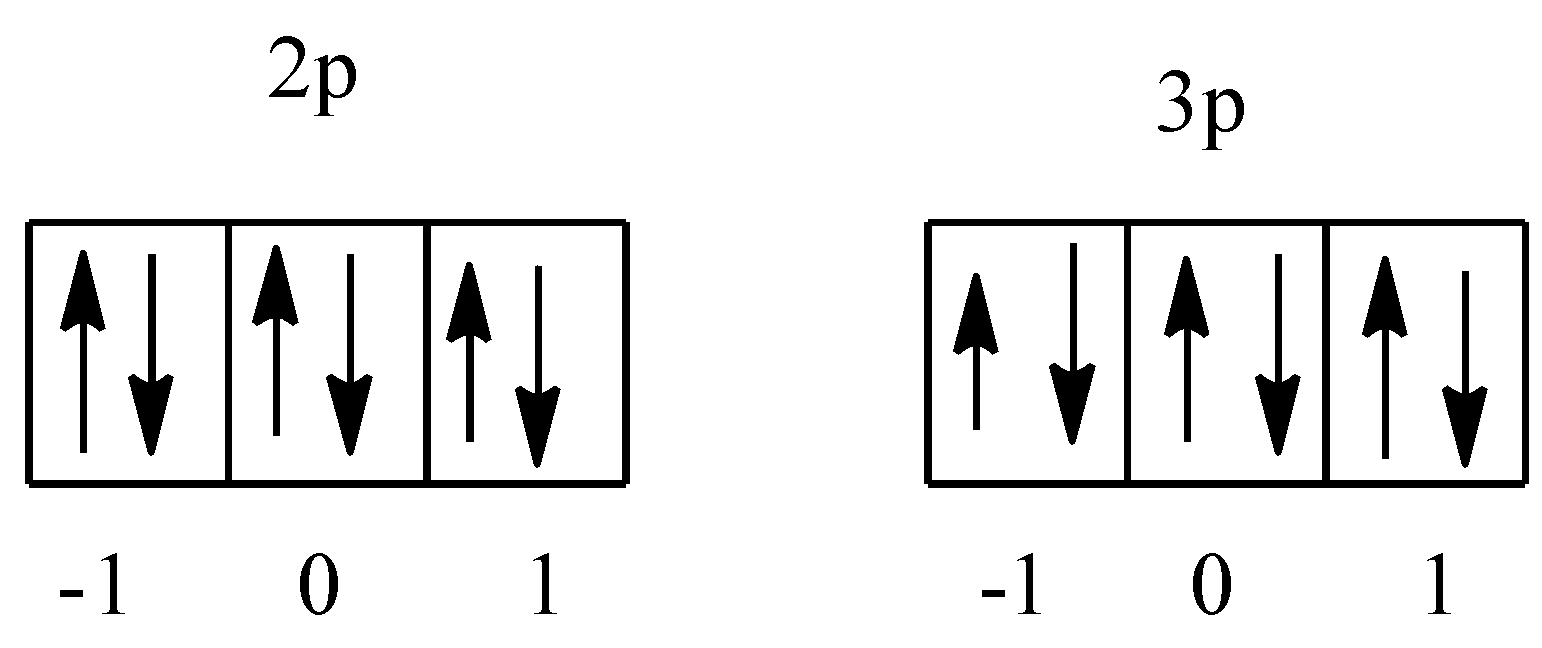
So, there are a total two orbital, i.e., one in each 2p and 3p having the value ${{m}_{l}}$= 1. In both the orbitals there are two electrons, therefore total electrons will be 4.
Hence the correct answer is an option (c)
Note:
The value of ${{m}_{l}}$= 1 will also come for d-subshell, but in argon it is not considered because in argon there is no d-subshell and no electrons into d-orbital. If the electron enters a d-subshell, then we have to count the electron in the d-subshell also.
Complete answer:
We know that there are four quantum numbers, i.e., principle quantum number, Azimuthal quantum number, magnetic quantum number, and spin quantum number.
${{m}_{l}}$ is the magnetic quantum number and l is the Azimuthal quantum number. With the help of l value we can find the magnetic quantum number. Given ${{m}_{l}}$=1, the values of the magnetic quantum number will range from -1 to 1.
The value of l is 1 when the subshell is p.
The given element in the question is Argon whose atomic number is 18 so, there are 18 electrons in argon atom. Its electronic configuration is given below:
$1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}$
We know that there are three orbitals in the p-subshell, which are denoted as -1, 0, and 1. In argon there are 2p and 3p orbitals. The arrangement of electrons is given below:

So, there are a total two orbital, i.e., one in each 2p and 3p having the value ${{m}_{l}}$= 1. In both the orbitals there are two electrons, therefore total electrons will be 4.
Hence the correct answer is an option (c)
Note:
The value of ${{m}_{l}}$= 1 will also come for d-subshell, but in argon it is not considered because in argon there is no d-subshell and no electrons into d-orbital. If the electron enters a d-subshell, then we have to count the electron in the d-subshell also.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




