
Consider the following structures.
Which of the following pairs represent D- and L-fructose respectively?
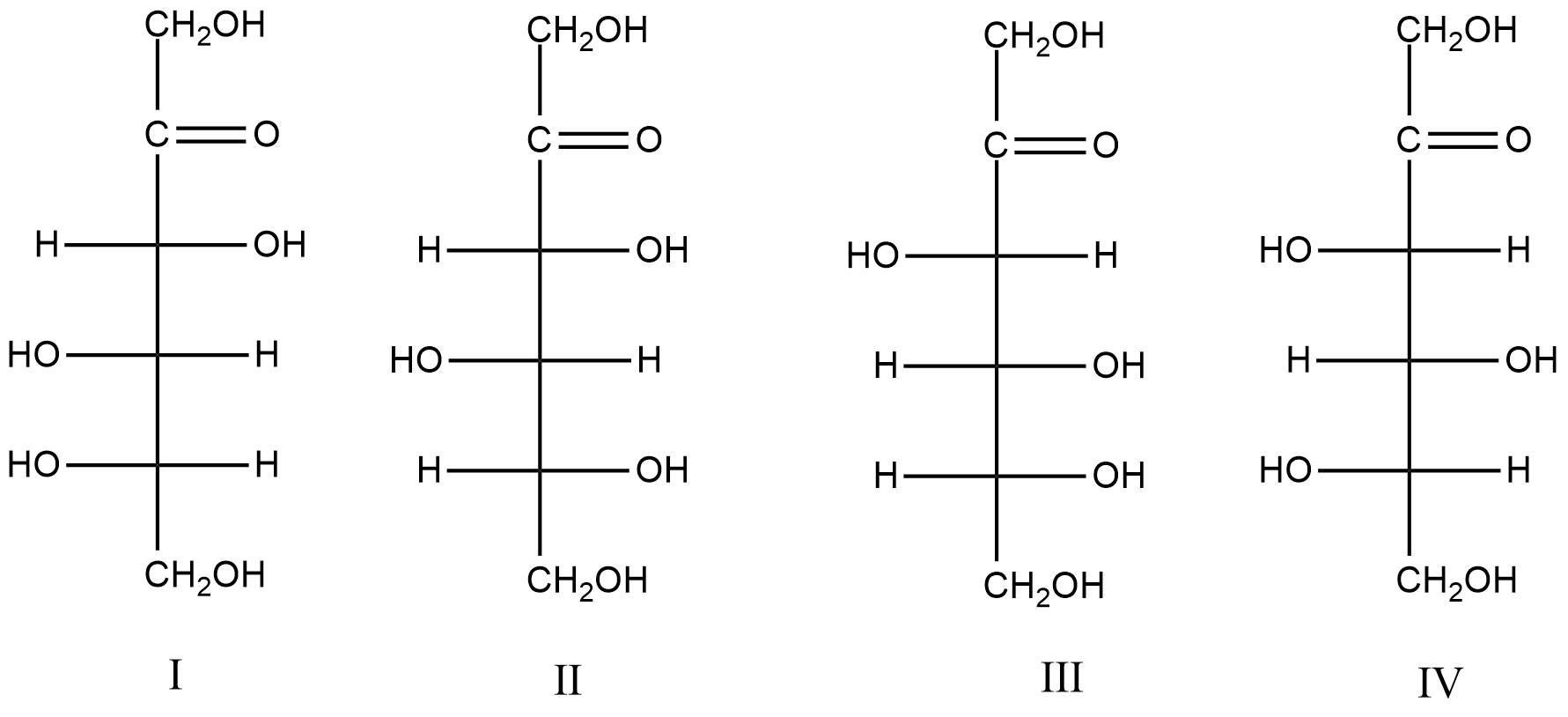
A. II and I
B. I and III
C. III and IV
D. II and IV

Answer
512.7k+ views
Hint: If a compound is going to rotate the plane polarized light to clockwise direction then the compound is called dextro form and if the compound is going to rotate the plane polarized light to anticlockwise direction then the compound is called leavo form.
Complete answer:
- In the question it is asked to find the D-fructose and L-fructose among the given four compounds.
- First, we should know the position of the hydroxyl groups on the carbon atoms of the fructose compound.
- Generally, the fructose is going to have two hydroxyl groups on carbon 4 and 5.
- These two carbons are going to decide whether the fructose is D form or L-form.
- The hydroxyl group on the 4th carbon should be on the right side only.
- Therefore, by considering the above parameters we can say that the structure III and IV are going to have D and L-form of fructose.
- The structures of D and L-fructose are as follows.
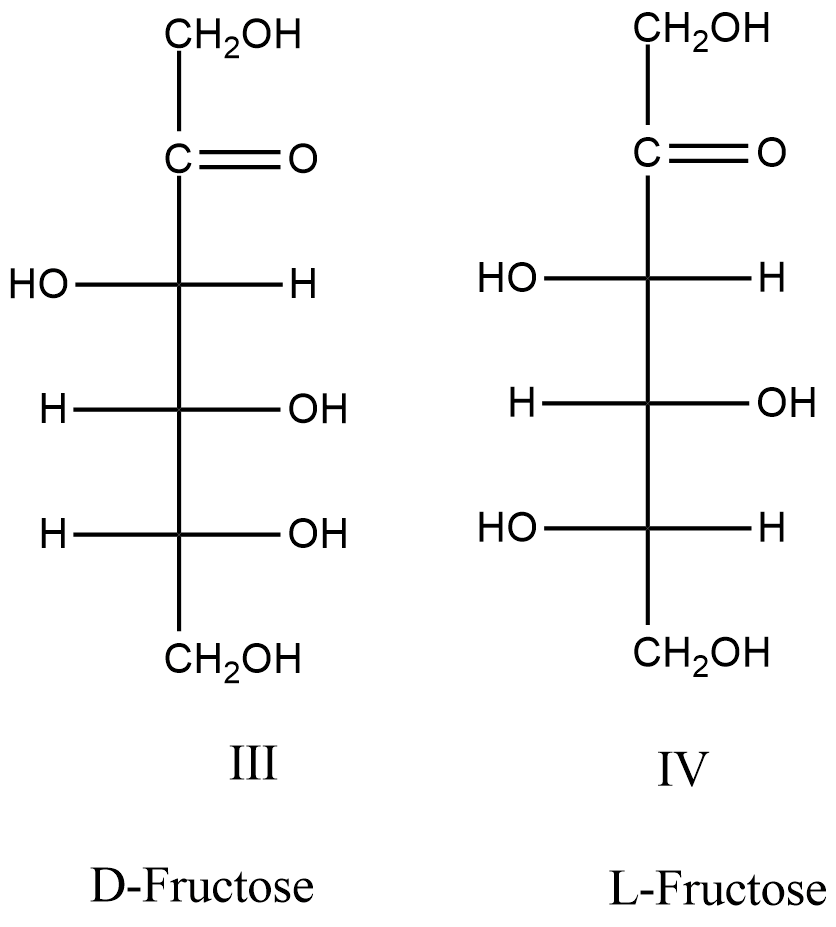
- Means the structures III and IV are going to represent the D-fructose and L-fructose structures.
So, the correct option is C.
Note:
D-fructose is going to rotate the polarized light to clockwise direction and the l-fructose is going to rotate the plane of polarized light to anticlockwise direction. The rotation of the molecule is going to decide the compound name.
Complete answer:
- In the question it is asked to find the D-fructose and L-fructose among the given four compounds.
- First, we should know the position of the hydroxyl groups on the carbon atoms of the fructose compound.
- Generally, the fructose is going to have two hydroxyl groups on carbon 4 and 5.
- These two carbons are going to decide whether the fructose is D form or L-form.
- The hydroxyl group on the 4th carbon should be on the right side only.
- Therefore, by considering the above parameters we can say that the structure III and IV are going to have D and L-form of fructose.
- The structures of D and L-fructose are as follows.

- Means the structures III and IV are going to represent the D-fructose and L-fructose structures.
So, the correct option is C.
Note:
D-fructose is going to rotate the polarized light to clockwise direction and the l-fructose is going to rotate the plane of polarized light to anticlockwise direction. The rotation of the molecule is going to decide the compound name.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE

How do I convert ms to kmh Give an example class 11 physics CBSE




