
How many constitutional isomers of ${C_7}{H_{15}}Cl$ will form in the radical chain chlorination of 2, 4-dimethylpentane.
Answer
553.2k+ views
Hint:Constitutional isomers also known as structural isomers are compounds which possess the same molecular formula but differ in their structural formula. In the compound ${C_7}{H_{15}}Cl$, three types of hydrogen bonding are present.
Complete step by step answer:Free radical halogenation reaction:
The free radical halogenation reaction is a type of halogenation reaction where single hydrogen of the alkane is replaced by the single halogen to form a haloalkane. This reaction takes place in presence of light.
This reaction has three steps.
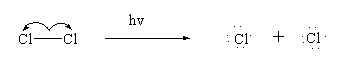
Step 1: Initiation
In this step the halogen molecule breaks down in presence of light to give free radicals.
Step 2: Propagation
The propagation step is divided further in two steps. In the first propagation step, the chlorine radical attacks the hydrogen atom present in the methane and forms hydrochloric acid and a methyl radical.
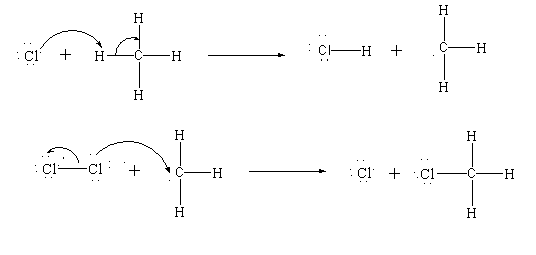
In the second propagation step more chlorine is added, out of which one chlorine atom becomes free radical and others get attached to the methyl radical.
Step 3: Termination
In the last step, the remaining free radicals combine with each other to generate new products like chloromethane and the two methyl radicals generate the by-product to form ethane.
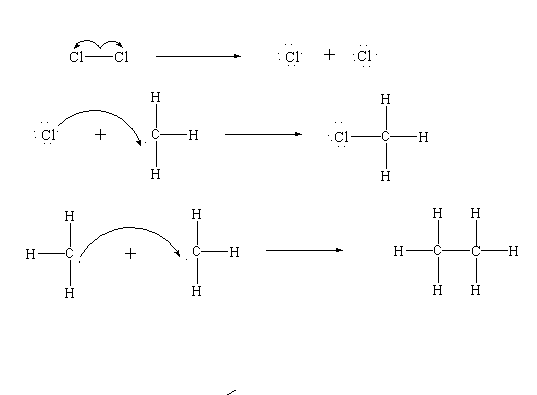
Note:
In free radical halogenation reaction, energy or light source is only needed in the first step that is initiation to break the halogen molecule into free radical. After that all the further reaction can be processed without any energy source.
Complete step by step answer:Free radical halogenation reaction:
The free radical halogenation reaction is a type of halogenation reaction where single hydrogen of the alkane is replaced by the single halogen to form a haloalkane. This reaction takes place in presence of light.
This reaction has three steps.
Step 1: Initiation
In this step the halogen molecule breaks down in presence of light to give free radicals.

Step 2: Propagation
The propagation step is divided further in two steps. In the first propagation step, the chlorine radical attacks the hydrogen atom present in the methane and forms hydrochloric acid and a methyl radical.

In the second propagation step more chlorine is added, out of which one chlorine atom becomes free radical and others get attached to the methyl radical.
Step 3: Termination
In the last step, the remaining free radicals combine with each other to generate new products like chloromethane and the two methyl radicals generate the by-product to form ethane.

Note:
In free radical halogenation reaction, energy or light source is only needed in the first step that is initiation to break the halogen molecule into free radical. After that all the further reaction can be processed without any energy source.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




