
Conversion of Acetic acid to Acetone.
Answer
527.7k+ views
Hint: We need to be sure about the structure of Acetic Acid and Acetone. The conversion of acetic acid directly into acetone, first changing it into some other compound, with neutralizing, can be figured out from the structure.
Complete answer:
The structure of Acetic Acid is:
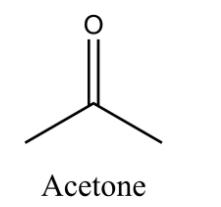
The structure of Acetone:
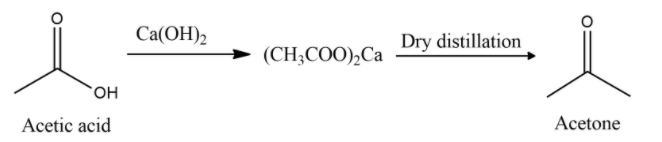
Here is the equation for the conversion of Acetic acid to acetone:
In the above equation we can see that Acetic acid is first converted to calcium salt when neutralizing it with quick lime. Calcium acetate on dry distillation yields Acetone by the decomposition. Acetone is usually prepared in the laboratory by the dry distillation of calcium acetate.
Note:
Here is some basic information that we should be knowing regarding the acetic acid and acetone.
Pure acetic acid is a colourless liquid with a strong corrosive pungent order; it has a characteristic of taste and is highly miscible in water. Acetic acid is largely used in the food industry as vinegar and is an acidity regulator. Acetic acid can be a hazardous chemical if it is not used in a safe and appropriate manner. This liquid is highly corrosive to the skin and eyes and because of this must be handled with extreme care acetic acid can also be damaging to the internal organs of unjust or in the case of vapour inhalation.
Acetone is the colourless mobile flammable liquid with a pleasant, somewhat fruity odour it is readily soluble in water, ethanol, ether, etc. and it serves as an important solvent. The physical properties of acetone such as high evaporation rate, low viscosity and miscibility with water and several organic solvents make it suitable for use as a solvent.
Complete answer:
The structure of Acetic Acid is:

The structure of Acetone:
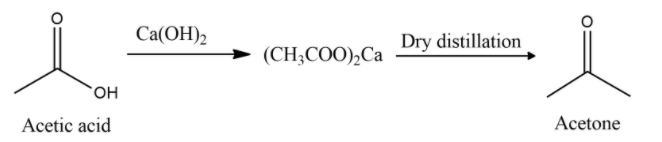
Here is the equation for the conversion of Acetic acid to acetone:
In the above equation we can see that Acetic acid is first converted to calcium salt when neutralizing it with quick lime. Calcium acetate on dry distillation yields Acetone by the decomposition. Acetone is usually prepared in the laboratory by the dry distillation of calcium acetate.
Note:
Here is some basic information that we should be knowing regarding the acetic acid and acetone.
Pure acetic acid is a colourless liquid with a strong corrosive pungent order; it has a characteristic of taste and is highly miscible in water. Acetic acid is largely used in the food industry as vinegar and is an acidity regulator. Acetic acid can be a hazardous chemical if it is not used in a safe and appropriate manner. This liquid is highly corrosive to the skin and eyes and because of this must be handled with extreme care acetic acid can also be damaging to the internal organs of unjust or in the case of vapour inhalation.
Acetone is the colourless mobile flammable liquid with a pleasant, somewhat fruity odour it is readily soluble in water, ethanol, ether, etc. and it serves as an important solvent. The physical properties of acetone such as high evaporation rate, low viscosity and miscibility with water and several organic solvents make it suitable for use as a solvent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




