
How will you convert acetone to chloroform?
Answer
584.1k+ views
Hint: Start by writing the structure of acetone and chloroform. Then mark the functional groups in both the compounds. Compare and see which all functional groups need to be removed and added to get acetone. Recollect the reagents used in chlorination since chloroform has chlorine atoms and one of the steps will involve chlorination. Write the reaction and then explain it.
Complete step by step answer:
- Acetone is the simplest ketone. Its IUPAC name is 2-propanone.
- Chloroform is a halogen derivative of methane having three chlorine atoms. It is also known as trichloromethane, $CHC{{l}_{3}}$.
- Acetone is a ketone and chloroform is an alkyl halide. So, from acetone we need to remove one acetyl group and two hydrogen atoms and add three chlorine atoms to get chloroform.
- Removing acetyl groups from acetone is difficult so first we need to chlorinate acetone to form 1, 1, 1-trichloro-2-propanone. Now, there are three chlorine atoms on one carbon atom making it electron deficient because chlorine is electronegative.
- So, now acetyl linkage can be easily broken in the presence of a base like calcium hydroxide. When calcium hydroxide is added to the intermediate 1, 1, 1-trichloro-2-propanone, calcium acetate is formed along with chloroform.
- The reaction is given as,
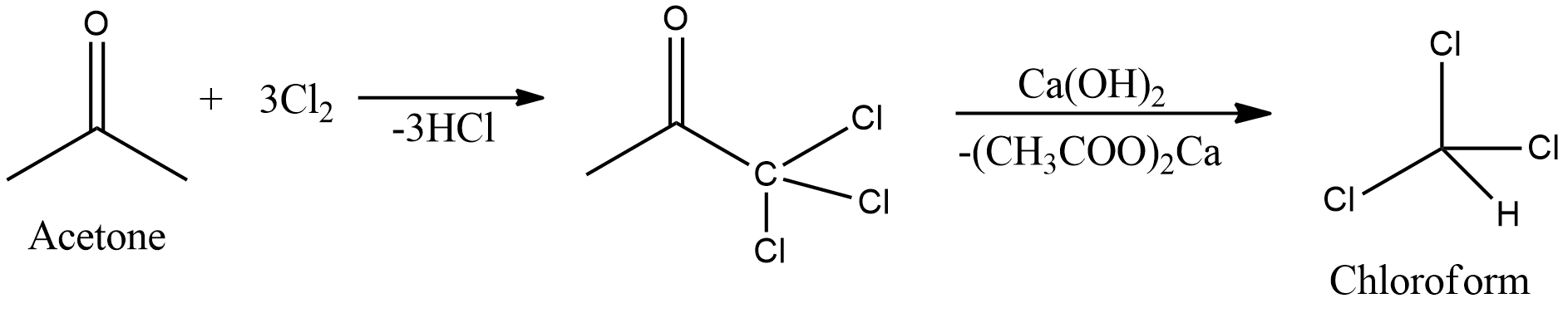
- Therefore, chloroform is formed from acetone by the first chlorination of acetone followed by action of calcium hydroxide.
Note: Remember chlorine is electronegative and therefore it will pull the shared electrons in covalent bond towards itself thus, creating polarity of bond. Because of this polarity, the bond between substituted carbon atoms and acetyl groups weakens. Calcium hydroxide being a base abstracts acetyl cation and trichlorocarban ion accepts a proton to form chloroform.
Complete step by step answer:
- Acetone is the simplest ketone. Its IUPAC name is 2-propanone.
- Chloroform is a halogen derivative of methane having three chlorine atoms. It is also known as trichloromethane, $CHC{{l}_{3}}$.
- Acetone is a ketone and chloroform is an alkyl halide. So, from acetone we need to remove one acetyl group and two hydrogen atoms and add three chlorine atoms to get chloroform.
- Removing acetyl groups from acetone is difficult so first we need to chlorinate acetone to form 1, 1, 1-trichloro-2-propanone. Now, there are three chlorine atoms on one carbon atom making it electron deficient because chlorine is electronegative.
- So, now acetyl linkage can be easily broken in the presence of a base like calcium hydroxide. When calcium hydroxide is added to the intermediate 1, 1, 1-trichloro-2-propanone, calcium acetate is formed along with chloroform.
- The reaction is given as,

- Therefore, chloroform is formed from acetone by the first chlorination of acetone followed by action of calcium hydroxide.
Note: Remember chlorine is electronegative and therefore it will pull the shared electrons in covalent bond towards itself thus, creating polarity of bond. Because of this polarity, the bond between substituted carbon atoms and acetyl groups weakens. Calcium hydroxide being a base abstracts acetyl cation and trichlorocarban ion accepts a proton to form chloroform.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




