
Convert : Aniline into 1, 3, 5- tribromo benzene.
Answer
560.7k+ views
Hint: The reaction of aniline to 1, 3, 5- tribromo benzene is an electrophilic substitution reaction in which bromo groups are substituted on a benzene ring and an amino group is removed by forming diazonium salt. First, the bromination occurs resulting in formation of 1, 3, 5- tribromo aniline and then the amino group is removed to give the product 1, 3, 5- tribromo benzene.
Complete step by step answer:
The aniline is a benzene ring having a -$N{H_2}$ group attached to it. The benzene is an aromatic compound. It is stabilised because of resonance. It does not undergo addition reactions because if it does, then the resonance of the ring is disturbed and it gets destabilised. Thus, it undergoes electrophilic substitution reaction. The reaction of aniline to 1, 3, 5- tribromo benzene is an electrophilic substitution reaction in which bromo groups are substituted on a benzene ring. The electrophile is $Br \oplus $ ion. The reaction takes place as- First, the bromine water reacts with aniline to give 1, 3, 5- tribromo aniline. Then, this product reacts with sodium nitrate and HCl to give diazonium salt. This reduction gives 1, 3, 5- tribromo benzene. The reaction can be drawn as -
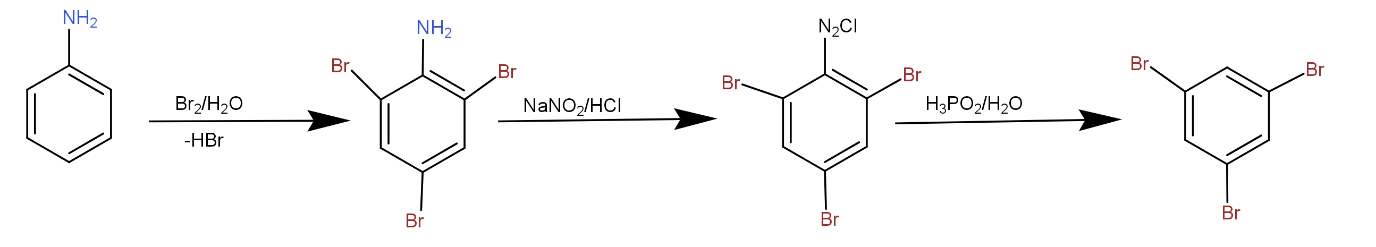
Note: The -$N{H_2}$ group is ortho para directing in nature. It directs the incoming groups to ortho and para positions of the benzene ring. So, it is not removed first. If it is removed first, the incoming group would attack at first carbon and the next two bromide ions will get substituted according to the first bromide ion.
Complete step by step answer:
The aniline is a benzene ring having a -$N{H_2}$ group attached to it. The benzene is an aromatic compound. It is stabilised because of resonance. It does not undergo addition reactions because if it does, then the resonance of the ring is disturbed and it gets destabilised. Thus, it undergoes electrophilic substitution reaction. The reaction of aniline to 1, 3, 5- tribromo benzene is an electrophilic substitution reaction in which bromo groups are substituted on a benzene ring. The electrophile is $Br \oplus $ ion. The reaction takes place as- First, the bromine water reacts with aniline to give 1, 3, 5- tribromo aniline. Then, this product reacts with sodium nitrate and HCl to give diazonium salt. This reduction gives 1, 3, 5- tribromo benzene. The reaction can be drawn as -

Note: The -$N{H_2}$ group is ortho para directing in nature. It directs the incoming groups to ortho and para positions of the benzene ring. So, it is not removed first. If it is removed first, the incoming group would attack at first carbon and the next two bromide ions will get substituted according to the first bromide ion.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




