
Convert Aniline into p-nitroaniline.
Answer
497.4k+ views
Hint: Aniline is an organic compound which consists of an amine functional group attached to the benzene ring while p-nitroaniline is also an organic compound in which the nitro functional group is attached at fourth position in Aniline. So, to convert Aniline into p-nitroaniline we would need to introduce a nitro group to the aniline. We will see the step by step conversion from aniline to p-nitroaniline in the solution.
Complete answer:
First we will see the structures of both aniline and p-nitroaniline.
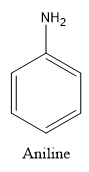
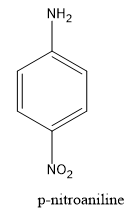
We know that aniline is an organic compound which consists of an amine functional group attached to the benzene ring while p-nitroaniline is also an organic compound in which the nitro functional group is attached at fourth position in Aniline.
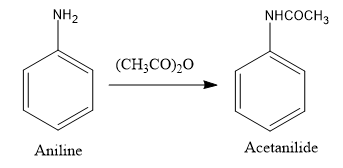
Now, to introduce nitro group in the aniline ring, the reagents used are $conc.HN{O_3}$ and $conc.{H_2}S{O_4}$ which are very strong acids. Amino group of aniline is a very strong base so it has great affinity towards these acids. Therefore, we need to protect amino group first by reacting aniline with acetic anhydride as follows:
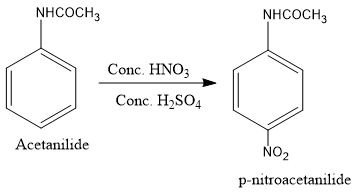
Then, the acetanilide formed is reacted with $conc.HN{O_3}$ and $conc.{H_2}S{O_4}$ to introduce nitro group at the para position. Nitro group is introduced at para position because the para position is the most active site as it has the highest electron density.
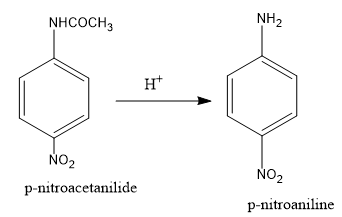
p-nitroacetanilide is hydrolysed to form p-nitroaniline.
Hence, in this way p-nitroaniline is synthesised from aniline.
Note:
We should remember that aniline is a very strong base and the amine group has great affinity towards the acid and if we do not protect the amine group it will react with the acids. Hence, the nitro group will not be introduced in the aniline ring. After introducing the nitro group in aniline ring, the amine group is deprotected by hydrolysis.
Complete answer:
First we will see the structures of both aniline and p-nitroaniline.


We know that aniline is an organic compound which consists of an amine functional group attached to the benzene ring while p-nitroaniline is also an organic compound in which the nitro functional group is attached at fourth position in Aniline.
Now, to introduce nitro group in the aniline ring, the reagents used are $conc.HN{O_3}$ and $conc.{H_2}S{O_4}$ which are very strong acids. Amino group of aniline is a very strong base so it has great affinity towards these acids. Therefore, we need to protect amino group first by reacting aniline with acetic anhydride as follows:

Then, the acetanilide formed is reacted with $conc.HN{O_3}$ and $conc.{H_2}S{O_4}$ to introduce nitro group at the para position. Nitro group is introduced at para position because the para position is the most active site as it has the highest electron density.

p-nitroacetanilide is hydrolysed to form p-nitroaniline.

Hence, in this way p-nitroaniline is synthesised from aniline.
Note:
We should remember that aniline is a very strong base and the amine group has great affinity towards the acid and if we do not protect the amine group it will react with the acids. Hence, the nitro group will not be introduced in the aniline ring. After introducing the nitro group in aniline ring, the amine group is deprotected by hydrolysis.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




