
How will you convert aniline to benzene?
Answer
573.9k+ views
Hint: Aniline is the common name for compound in which ${\text{ - N}}{{\text{H}}_{\text{2}}}$ functional group is attached with benzene. Since \[{\text{ - N}}{{\text{H}}_{\text{2}}}\] group is not a good leaving group so we convert it into a good leaving group by diazotizing it.
Complete step by step answer:
First we will convert the aniline to benzene diazonium chloride to make amine a better leaving group

Aniline Benzene diazonium chloride
This is the diazotization of the amine group into diazonium salt with the help of \[{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{ + HCl}}\] at \[{\text{0^\circ C}}\]
This step is the most important step.
Now we will react this benzene diazonium chloride with hypophosphorous acid to convert it to benzene.
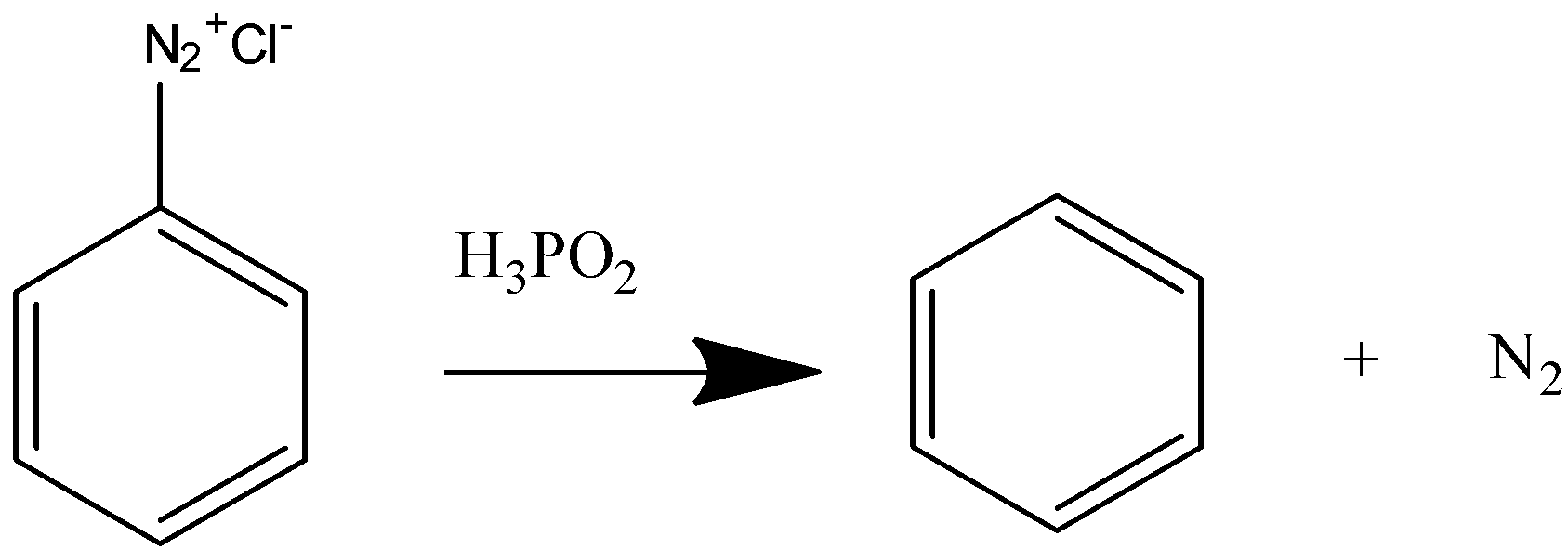
On reacting with hypophosphorous acid the diazonium salt releases nitrogen gas and benzene is formed.
In the above reaction the first step is very important as it increases the leaving capacity of nitrogen. The reagents \[{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{ + HCl}}\] gives \[{\text{HN}}{{\text{O}}_{\text{2}}}\] which converts the \[{\text{ - N}}{{\text{H}}_{\text{2}}}\] group into oxime group \[{\text{ - N = N - OH}}\]
This oxime is unstable and reacts with remaining \[{\text{HCl}}\] in the container to form \[{{\text{N}}_{\text{2}}}^{\text{ + }}{\text{C}}{{\text{l}}^{\text{ - }}}\] which is called diazonium chloride. This diazonium can react with a variety of different reagents to form a large variety of different products from those compounds who have poor leaving groups. The poor leaving group converts to a very good leaving group like diazonium chloride and forms a product further.
Note: After diazotization the benzene diazonium chloride salt can be converted to fluoro-benzene by Schiemann’s reaction , chloro-benzene by Sandmeyer reaction , iodo-benzene by Gattermann reaction, benzene by hypo-phosphorus acid etc.
The mixture of \[{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{ + HCl}}\] should be freshly prepared so that only \[{\text{HN}}{{\text{O}}_{\text{2}}}\] is formed otherwise it will convert to \[{\text{HN}}{{\text{O}}_{\text{3}}}\] nitric acid which cannot be a nucleophile for this reaction.
Complete step by step answer:
First we will convert the aniline to benzene diazonium chloride to make amine a better leaving group

Aniline Benzene diazonium chloride
This is the diazotization of the amine group into diazonium salt with the help of \[{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{ + HCl}}\] at \[{\text{0^\circ C}}\]
This step is the most important step.
Now we will react this benzene diazonium chloride with hypophosphorous acid to convert it to benzene.

On reacting with hypophosphorous acid the diazonium salt releases nitrogen gas and benzene is formed.
In the above reaction the first step is very important as it increases the leaving capacity of nitrogen. The reagents \[{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{ + HCl}}\] gives \[{\text{HN}}{{\text{O}}_{\text{2}}}\] which converts the \[{\text{ - N}}{{\text{H}}_{\text{2}}}\] group into oxime group \[{\text{ - N = N - OH}}\]
This oxime is unstable and reacts with remaining \[{\text{HCl}}\] in the container to form \[{{\text{N}}_{\text{2}}}^{\text{ + }}{\text{C}}{{\text{l}}^{\text{ - }}}\] which is called diazonium chloride. This diazonium can react with a variety of different reagents to form a large variety of different products from those compounds who have poor leaving groups. The poor leaving group converts to a very good leaving group like diazonium chloride and forms a product further.
Note: After diazotization the benzene diazonium chloride salt can be converted to fluoro-benzene by Schiemann’s reaction , chloro-benzene by Sandmeyer reaction , iodo-benzene by Gattermann reaction, benzene by hypo-phosphorus acid etc.
The mixture of \[{\text{NaN}}{{\text{O}}_{\text{2}}}{\text{ + HCl}}\] should be freshly prepared so that only \[{\text{HN}}{{\text{O}}_{\text{2}}}\] is formed otherwise it will convert to \[{\text{HN}}{{\text{O}}_{\text{3}}}\] nitric acid which cannot be a nucleophile for this reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




