
Convert benzaldehyde to benzophenone.
Answer
527k+ views
Hint: When we do the oxidation of aldehyde, it is converted into carboxylic acid. And then by decarboxylation it gets converted to aromatic hydrocarbon. Then if we treat it with benzoyl chloride, we get the desired result.
Complete step-by-step answer:
First, we should know about benzaldehyde. So, benzaldehyde (\[{{C}_{6}}{{H}_{5}}CHO\]) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
We should know its physical properties that it is a colourless liquid with a characteristic almond-like odour. The primary component of bitter almond oil, benzaldehyde can be extracted from a number of other natural sources. We use synthetic benzaldehyde for flavoring cakes.
Now, we should know about benzoic acid. So, benzoic acid is a white (or colourless) solid with the formula \[{{C}_{6}}{{H}_{5}}COOH\]. We use benzoic acid most commonly in industrial settings to manufacture a wide variety of products such as perfumes, dyes, topical medications and insect repellents.
So, now we will focus on our question: the conversion of benzaldehyde to benzoic acid.
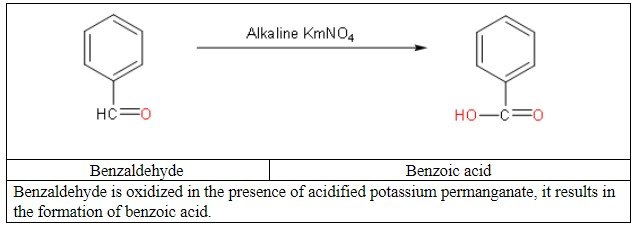
Then convert benzoic acid to benzene.
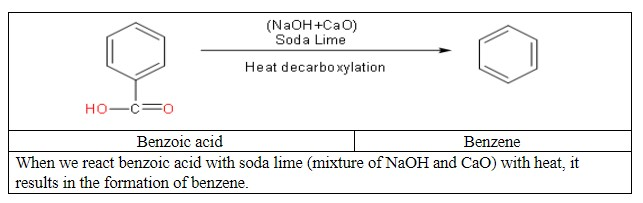
Then with the final step we will convert benzene to benzophenone.
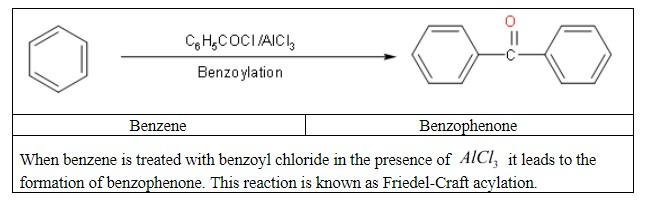
Note: We should know about Friedel–Crafts reactions. These are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts acylation involves the acylation of aromatic rings. We use typical acylating agents such as acyl chlorides.
Complete step-by-step answer:
First, we should know about benzaldehyde. So, benzaldehyde (\[{{C}_{6}}{{H}_{5}}CHO\]) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
We should know its physical properties that it is a colourless liquid with a characteristic almond-like odour. The primary component of bitter almond oil, benzaldehyde can be extracted from a number of other natural sources. We use synthetic benzaldehyde for flavoring cakes.
Now, we should know about benzoic acid. So, benzoic acid is a white (or colourless) solid with the formula \[{{C}_{6}}{{H}_{5}}COOH\]. We use benzoic acid most commonly in industrial settings to manufacture a wide variety of products such as perfumes, dyes, topical medications and insect repellents.
So, now we will focus on our question: the conversion of benzaldehyde to benzoic acid.

Then convert benzoic acid to benzene.

Then with the final step we will convert benzene to benzophenone.

Note: We should know about Friedel–Crafts reactions. These are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts acylation involves the acylation of aromatic rings. We use typical acylating agents such as acyl chlorides.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




