
Convert benzene to benzyl alcohol.
Answer
559.2k+ views
Hint: Benzyl alcohol is an organic compound in which a hydroxyl group is attached to a $ - {{C}}{{{H}}_2}$ group which is attached to the benzene ring. This cannot be done in one step. It involves several processes and formation of different compounds.
Complete step by step answer:
The structures of benzene and benzyl alcohol are given below:
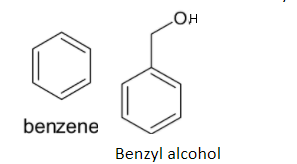
Benzene is a very stable organic compound. Thus it is very difficult for the direct substitution of the hydroxyl group. So it takes place in several steps:
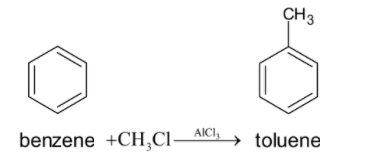
First we have to do the Friedel-Crafts alkylation reaction of benzene. This is done by reacting the benzene with ${{C}}{{{H}}_3}{{Cl}}$ in the presence of aluminium chloride as catalyst. So the methyl group gets substituted in the benzene ring. The chemical reaction is given below:
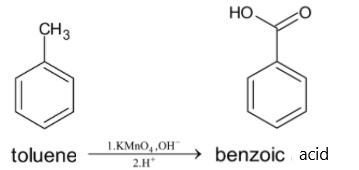
Now this toluene is oxidized with potassium permanganate solution to give benzoic acid. The methyl group outside the benzene ring gets oxidized to form a carboxylic acid. This reaction must be in the basic medium followed by the addition of acidic hydrogen ions. We can use any oxidizing agents, but for oxidation of benzene, ${{KMn}}{{{O}}_4}$ is used.
Now the benzoic acid has to be reduced to get benzyl alcohol. We have to use a suitable reducing agent like ${{LiAL}}{{{H}}_4}$.
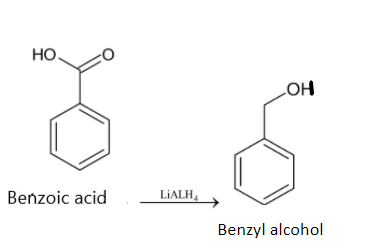
Thus benzene is converted to benzyl alcohol.
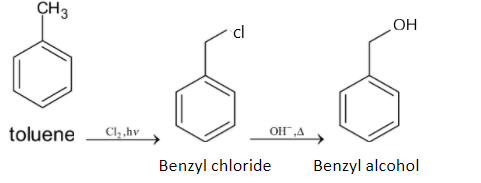
Note: There is another method for converting toluene to benzyl alcohol. Toluene is chlorinated first and then it is heated with aqueous sodium hydroxide or potassium hydroxide. When toluene is chlorinated, it forms benzyl chloride and then it forms benzyl alcohol when it reacts with sodium hydroxide or potassium hydroxide. The chemical reaction is given below:
Complete step by step answer:
The structures of benzene and benzyl alcohol are given below:

Benzene is a very stable organic compound. Thus it is very difficult for the direct substitution of the hydroxyl group. So it takes place in several steps:
First we have to do the Friedel-Crafts alkylation reaction of benzene. This is done by reacting the benzene with ${{C}}{{{H}}_3}{{Cl}}$ in the presence of aluminium chloride as catalyst. So the methyl group gets substituted in the benzene ring. The chemical reaction is given below:

Now this toluene is oxidized with potassium permanganate solution to give benzoic acid. The methyl group outside the benzene ring gets oxidized to form a carboxylic acid. This reaction must be in the basic medium followed by the addition of acidic hydrogen ions. We can use any oxidizing agents, but for oxidation of benzene, ${{KMn}}{{{O}}_4}$ is used.

Now the benzoic acid has to be reduced to get benzyl alcohol. We have to use a suitable reducing agent like ${{LiAL}}{{{H}}_4}$.

Thus benzene is converted to benzyl alcohol.
Note: There is another method for converting toluene to benzyl alcohol. Toluene is chlorinated first and then it is heated with aqueous sodium hydroxide or potassium hydroxide. When toluene is chlorinated, it forms benzyl chloride and then it forms benzyl alcohol when it reacts with sodium hydroxide or potassium hydroxide. The chemical reaction is given below:

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




