
How will you convert Benzene to biphenyl?
Answer
523k+ views
Hint: Benzene can be converted to chlorobenzene by using \[C{{l}_{2}}+FeC{{l}_{3}}\] or \[AlC{{l}_{3}}\] and then perform the Fitting reaction of chlorobenzene which gives biphenyl as a product.
Complete answer:
There are two steps we use here for this conversion, first is benzene to chlorobenzene and second chlorobenzene to biphenyl (as a final product or desired product). For the first conversion we take \[C{{l}_{2}}+AlC{{l}_{3}}\](anhydrous) and this is called a chlorination reaction. It is an electrophilic substitution. Here chlorination reaction of benzene is as follows:
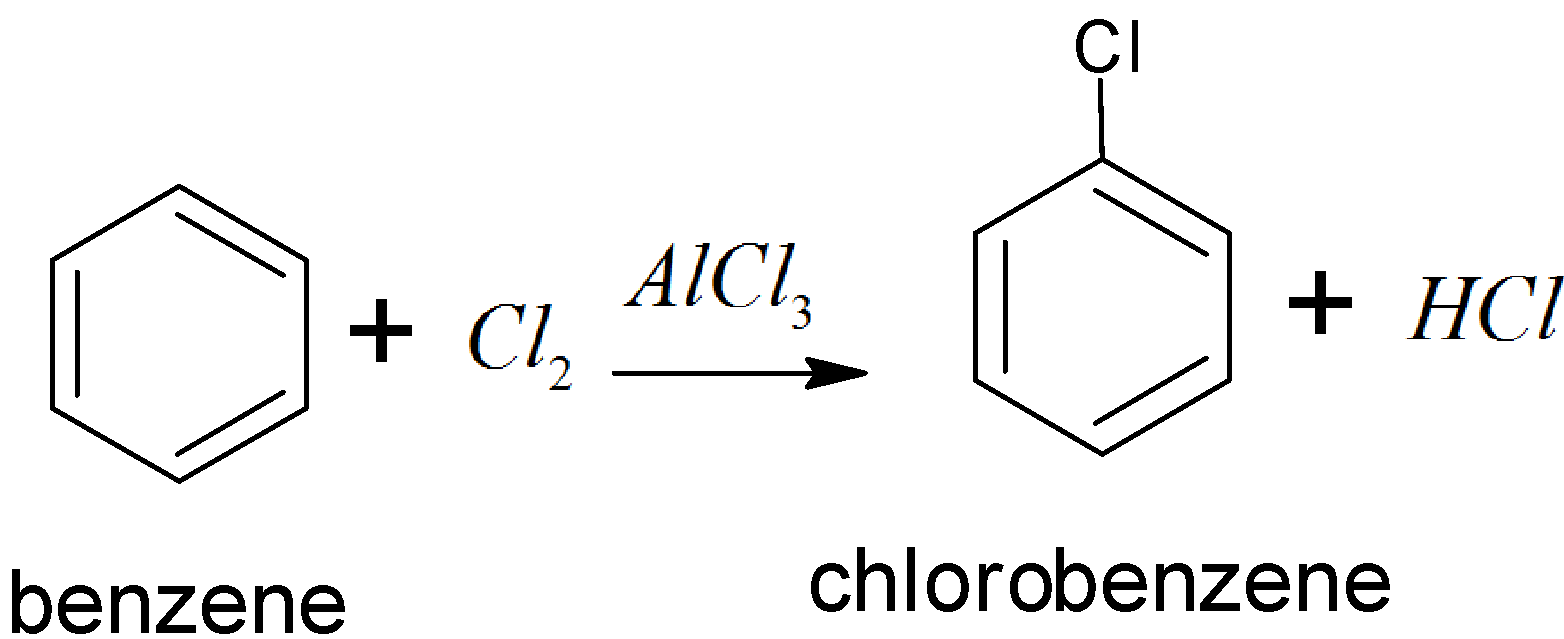
Then in step two we have to convert chlorobenzene to biphenyl in the presence of sodium and dry ether. This reaction is known as the fitting reaction. And the reaction is as follows:
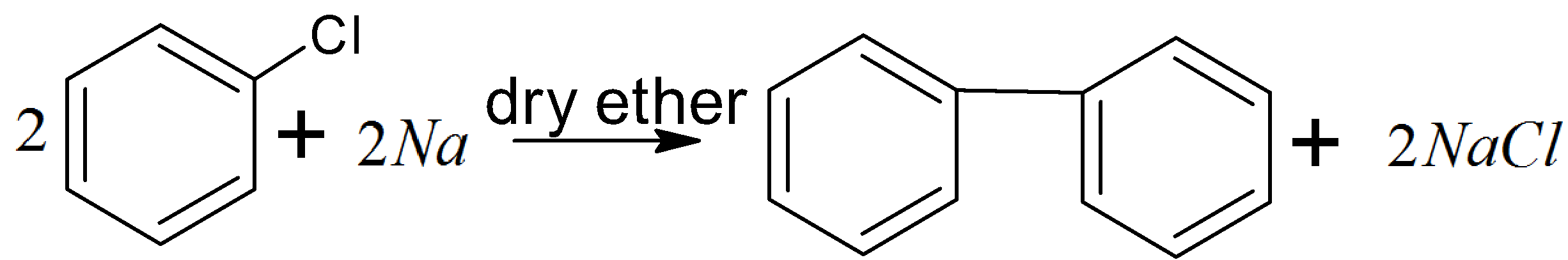
There is another method also like Ullmann’s reaction in which chlorobenzene is treated with Cu metal and then biphenyl is obtained. And the reaction mechanism is as follows:
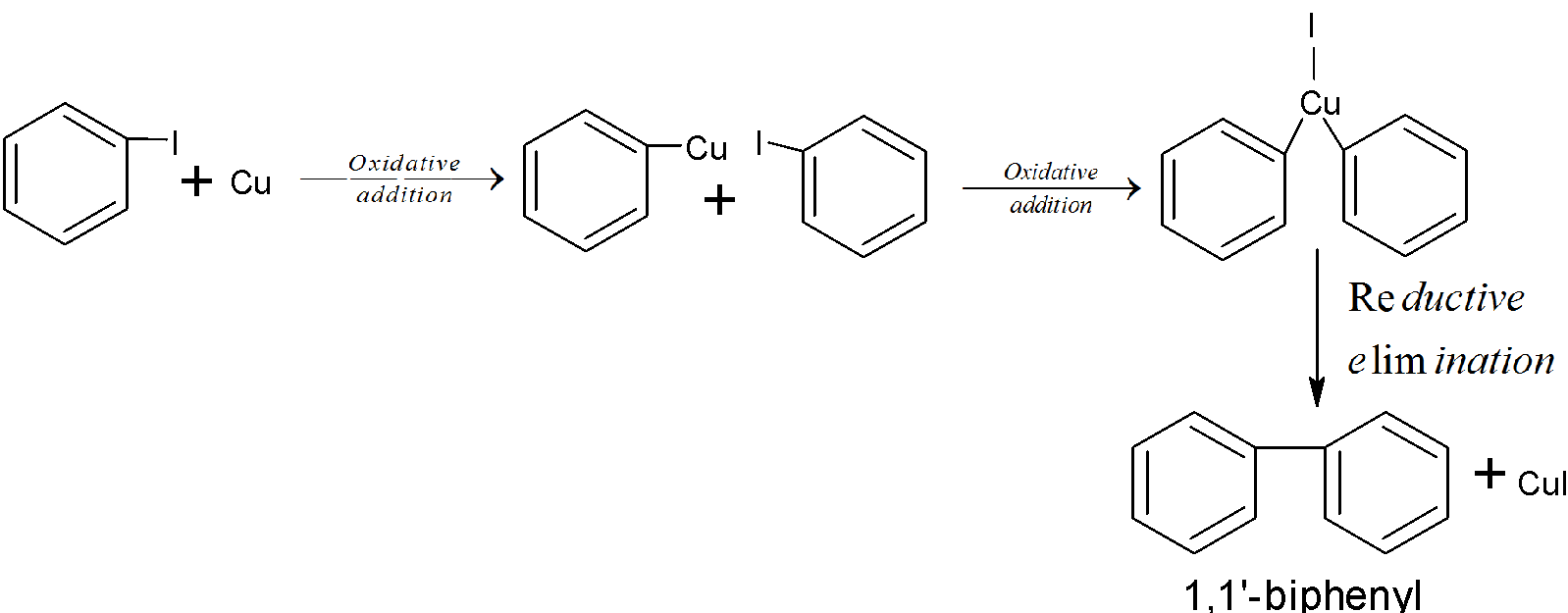
There are many types of reactions mechanisms are involved in this like elimination reaction, substitution reaction, addition reaction etc.
Note:
We should know the mechanism steps involved in these methods, so that we don’t make any mistakes in breaking bonds or addition of any element. In this mechanism another halogens can also be used.
Complete answer:
There are two steps we use here for this conversion, first is benzene to chlorobenzene and second chlorobenzene to biphenyl (as a final product or desired product). For the first conversion we take \[C{{l}_{2}}+AlC{{l}_{3}}\](anhydrous) and this is called a chlorination reaction. It is an electrophilic substitution. Here chlorination reaction of benzene is as follows:
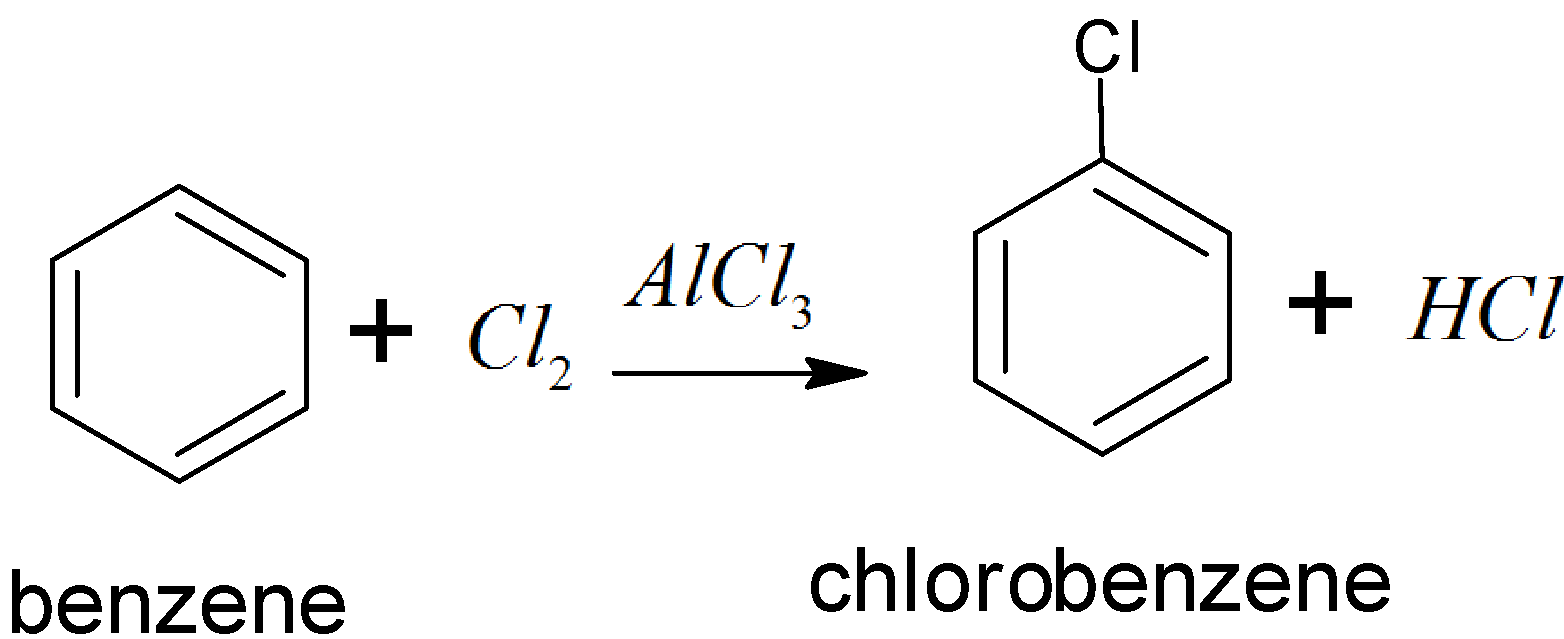
Then in step two we have to convert chlorobenzene to biphenyl in the presence of sodium and dry ether. This reaction is known as the fitting reaction. And the reaction is as follows:

There is another method also like Ullmann’s reaction in which chlorobenzene is treated with Cu metal and then biphenyl is obtained. And the reaction mechanism is as follows:

There are many types of reactions mechanisms are involved in this like elimination reaction, substitution reaction, addition reaction etc.
Note:
We should know the mechanism steps involved in these methods, so that we don’t make any mistakes in breaking bonds or addition of any element. In this mechanism another halogens can also be used.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




