
How to convert phenol to benzoquinone?
Answer
540k+ views
Hint: To solve the conversion we should quickly recollect the various reactions in organic chemistry. Here the given molecule is phenol and is getting converted to benzoquinone so an oxidation reaction should be carried out to yield the desired result.
Complete step-by-step answer:In the question, a conversion reaction is given and we have to analyse which reaction should be carried out to get the desired result and identify the chemicals used in the chemical reaction.
In the question phenol is given, we know that phenol is an aromatic hydrocarbon which has a benzene ring and a C of benzene ring attached to –OH functional group, ie phenol is an alcohol.
The structure of phenol is as follows:
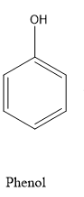
And the product that should be formed is benzoquinone. Quinone with one benzene ring is called a benzoquinone structure, it will have two ketonic groups which are para to each other. And hence the structure to be formed is 1, 4-benzoquinone and the structure is as follows:
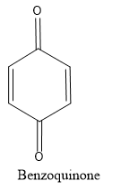
In the quinone structure there is a ketonic group instead of an alcohol group, hence the reaction that should be carried out should be an oxidation reaction.
So we can use oxidizing agents to convert phenol to benzoquinone.
Since alcohols can be converted into ketones and aldehydes by carrying out oxidation reactions according to the type of alcohol present.
So here the phenol is a secondary carbon and the alcoholic group will be converted to a ketonic group using an oxidation reaction.
But in the structure of benzoquinone an extra O is present so we should use a strong oxidizing agent to carry out the reaction.
We use sodium dichromate along with sulphuric acid to convert phenol to benzoquinone.
The reaction is as follows:

Note:Using an oxidizing agent if we carry out an oxidation reaction with primary alcohol then an aldehyde will be formed. If we use oxidizing agents like potassium permanganate, potassium dichromate then the product will be ketone and will have only one oxygen atom, whereas in the benzoquinone two ketonic groups are present.
Two structures of benzoquinone are possible- 1, 2-benzoquinone and 1,4-benzoquinone but the most common one is 1,4-benzoquinone.
Complete step-by-step answer:In the question, a conversion reaction is given and we have to analyse which reaction should be carried out to get the desired result and identify the chemicals used in the chemical reaction.
In the question phenol is given, we know that phenol is an aromatic hydrocarbon which has a benzene ring and a C of benzene ring attached to –OH functional group, ie phenol is an alcohol.
The structure of phenol is as follows:

And the product that should be formed is benzoquinone. Quinone with one benzene ring is called a benzoquinone structure, it will have two ketonic groups which are para to each other. And hence the structure to be formed is 1, 4-benzoquinone and the structure is as follows:

In the quinone structure there is a ketonic group instead of an alcohol group, hence the reaction that should be carried out should be an oxidation reaction.
So we can use oxidizing agents to convert phenol to benzoquinone.
Since alcohols can be converted into ketones and aldehydes by carrying out oxidation reactions according to the type of alcohol present.
So here the phenol is a secondary carbon and the alcoholic group will be converted to a ketonic group using an oxidation reaction.
But in the structure of benzoquinone an extra O is present so we should use a strong oxidizing agent to carry out the reaction.
We use sodium dichromate along with sulphuric acid to convert phenol to benzoquinone.
The reaction is as follows:

Note:Using an oxidizing agent if we carry out an oxidation reaction with primary alcohol then an aldehyde will be formed. If we use oxidizing agents like potassium permanganate, potassium dichromate then the product will be ketone and will have only one oxygen atom, whereas in the benzoquinone two ketonic groups are present.
Two structures of benzoquinone are possible- 1, 2-benzoquinone and 1,4-benzoquinone but the most common one is 1,4-benzoquinone.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




