
How do you convert the following?
Toluene to Benzoic acid
Answer
524.8k+ views
Hint: We must know that the oxidation of toluene using suitable oxidizing agent will give you benzoic acid.Toluene,also known as methylbenzene and its chemical formula is $C_7$$H_8$ whereas Benzene’s chemical formula is $C_6$$H_6$.
Complete step by step solution:
We can prepare benzoic acid from toluene by laboratory process.
Therefore, Mix 3 ml of Toluene with 10 gm of Potassium Permanganate and 20 ml of dilute solution of sodium hydroxide in round bottom flask
Set up the reflux and start mixture to reflux for 3 to 4 hours until oily toluene disappears.
Cool down the solution and filter out the manganese oxide from the solution.
Add concentrated \[HCl\] to the filtrate
Collect the precipitate of benzoic acid and recrystallize using hot \[{H_2}O\], or can extract the benzoic acid with ether.
We can write the above process in chemical equation form as below.
Toluene should be heated in presence of alkaline \[KMn{O_4}\] to carry out oxidation of Toluene. This process oxidizes toluene into potassium benzoate ions.
Potassium benzoate ions are then followed by acidification to form benzoic acid.
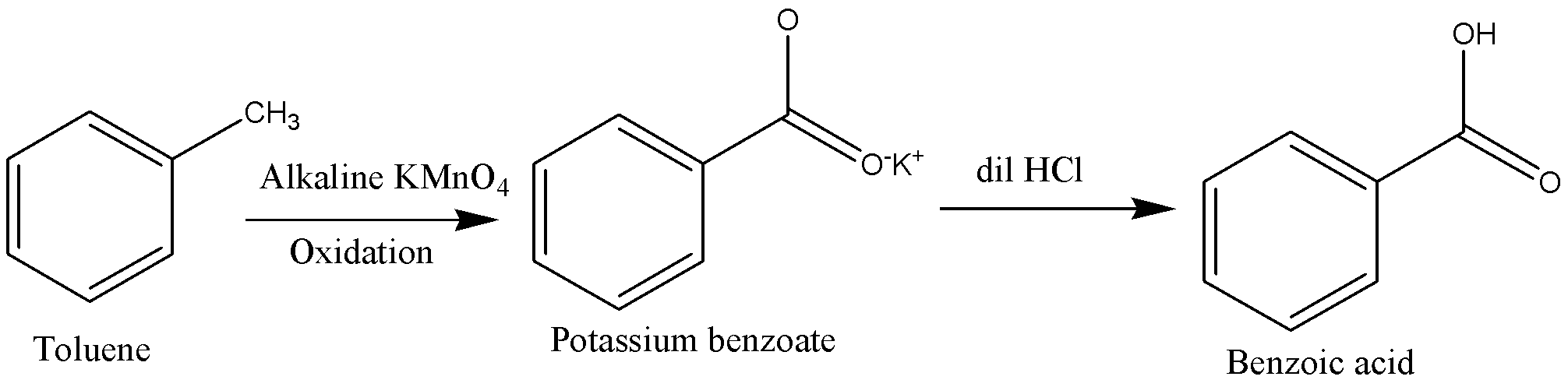
We will reagents such as acidic potassium permanganate \[\left( {KMn{O_4}} \right)\], Acidic potassium dichromate \[\left( {{K_2}C{r_2}{O_7}} \right)\] and dilute\[HN{O_3}\]. Other oxidizing agents also can use in conversion of toluene into benzoic acid.
This is a simple oxidation method where the Methyl group gets converted to a carboxylic group using potassium permanganate as an oxidizing agent. Potassium hydroxide provides an alkaline medium because strong oxidizing agents are stable only under alkaline medium. \[HCl\] addition neutralizes the added \[KOH\]and then converts potassium benzoate in the filtrate into benzoic acid.
Note: Even though there are many different ways to prepare Benzoic acid from Toluene, the simple one is mentioned above.Under neutral conditions, \[M{n^{ + 7}}\]is reduced to \[M{n^{ + 4}}\]and under acidic conditions it is reduced to\[M{n^{ + 2}}\]. Both neutral and acidic medium conditions make \[KMn{O_4}\]less powerful oxidizing agents.
Complete step by step solution:
We can prepare benzoic acid from toluene by laboratory process.
Therefore, Mix 3 ml of Toluene with 10 gm of Potassium Permanganate and 20 ml of dilute solution of sodium hydroxide in round bottom flask
Set up the reflux and start mixture to reflux for 3 to 4 hours until oily toluene disappears.
Cool down the solution and filter out the manganese oxide from the solution.
Add concentrated \[HCl\] to the filtrate
Collect the precipitate of benzoic acid and recrystallize using hot \[{H_2}O\], or can extract the benzoic acid with ether.
We can write the above process in chemical equation form as below.
Toluene should be heated in presence of alkaline \[KMn{O_4}\] to carry out oxidation of Toluene. This process oxidizes toluene into potassium benzoate ions.
Potassium benzoate ions are then followed by acidification to form benzoic acid.

We will reagents such as acidic potassium permanganate \[\left( {KMn{O_4}} \right)\], Acidic potassium dichromate \[\left( {{K_2}C{r_2}{O_7}} \right)\] and dilute\[HN{O_3}\]. Other oxidizing agents also can use in conversion of toluene into benzoic acid.
This is a simple oxidation method where the Methyl group gets converted to a carboxylic group using potassium permanganate as an oxidizing agent. Potassium hydroxide provides an alkaline medium because strong oxidizing agents are stable only under alkaline medium. \[HCl\] addition neutralizes the added \[KOH\]and then converts potassium benzoate in the filtrate into benzoic acid.
Note: Even though there are many different ways to prepare Benzoic acid from Toluene, the simple one is mentioned above.Under neutral conditions, \[M{n^{ + 7}}\]is reduced to \[M{n^{ + 4}}\]and under acidic conditions it is reduced to\[M{n^{ + 2}}\]. Both neutral and acidic medium conditions make \[KMn{O_4}\]less powerful oxidizing agents.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




