
Convert toluene into benzene in 2 steps reaction.
Answer
569.7k+ views
Hint: Toluene is an organic aromatic compound having a methyl group present on the carbon atom on the benzene ring. First, oxidize the toluene with hot potassium manganate solution and then react it with sodium hydroxide or calcium oxide.
Complete answer:
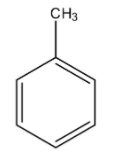
Toluene is an organic aromatic compound in which the methyl group is present on one of the carbon atoms of the benzene ring. The formula of toluene is ${{C}_{6}}{{H}_{5}}C{{H}_{3}}$. The structure of the toluene is given below:
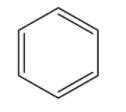
Benzene is an aromatic ring of six carbon atoms having three double bonds. The formula of benzene is ${{C}_{6}}{{H}_{6}}$. The structure of the benzene is given below:
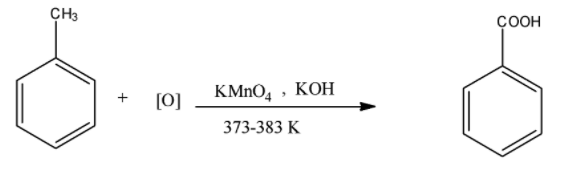
For converting the toluene to benzene in 2 steps, first, convert toluene to benzoic acid and then convert this benzoic acid to benzene.
When toluene is oxidized with hot potassium manganate solution and potassium hydroxide at 373 – 383 K, there is the formation of benzoic acid. Benzoic acid is an organic aromatic compound in which the carbon atom of the benzene ring is attached with the $-COOH$ group. The formula of benzoic acid is ${{C}_{6}}{{H}_{5}}COOH$. The reaction is given below:
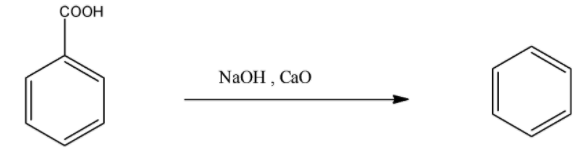
Now, this benzoic acid can be converted into benzene, when the benzoic acid is heated with sodium hydroxide or calcium oxide. The reaction is given below:
So by these two steps, toluene can be converted into benzene.
Note:
When the aromatic compounds having methyl group on the carbon atoms of the benzene ring is oxidized with hot potassium manganate solution, all the methyl groups convert into the acid group, even there is more than one methyl is present on the benzene ring.
Complete answer:
Toluene is an organic aromatic compound in which the methyl group is present on one of the carbon atoms of the benzene ring. The formula of toluene is ${{C}_{6}}{{H}_{5}}C{{H}_{3}}$. The structure of the toluene is given below:

Benzene is an aromatic ring of six carbon atoms having three double bonds. The formula of benzene is ${{C}_{6}}{{H}_{6}}$. The structure of the benzene is given below:

For converting the toluene to benzene in 2 steps, first, convert toluene to benzoic acid and then convert this benzoic acid to benzene.
When toluene is oxidized with hot potassium manganate solution and potassium hydroxide at 373 – 383 K, there is the formation of benzoic acid. Benzoic acid is an organic aromatic compound in which the carbon atom of the benzene ring is attached with the $-COOH$ group. The formula of benzoic acid is ${{C}_{6}}{{H}_{5}}COOH$. The reaction is given below:

Now, this benzoic acid can be converted into benzene, when the benzoic acid is heated with sodium hydroxide or calcium oxide. The reaction is given below:

So by these two steps, toluene can be converted into benzene.
Note:
When the aromatic compounds having methyl group on the carbon atoms of the benzene ring is oxidized with hot potassium manganate solution, all the methyl groups convert into the acid group, even there is more than one methyl is present on the benzene ring.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




