
Copper crystallises into a fcc lattice with edges length \[3.61\]×${10^{ - 8}}$cm. Show that the calculated density is in agreement with its measured value of $8.92g$$c{m^3}$
Answer
576.9k+ views
Hint: We know that density is the mean mass of a unit volume of a substance.
We must remember that an appointment of atoms in crystals during which the atomic centers are disposed in space is such that one atom is found at each of the corners of the cube and one at the middle of every face.
We know that in FCC, there are eight atoms at each corner (each shared by 8 unit cells) and six at the faces (each shared by two unit cells).
The number of atoms per unit cell in FCC is $8 \times (1/8) + 6 \times (1/2) = 4$ .
Formula Used:The formula of density is given as below,
$D = \dfrac{{ZM}}{{{a^3}{N_A}}}$
Where,
Z = No. of atoms in unit cell
M = Atomic mass of the substance
a = Edge length
$N_A$ = Avogadro number
Complete step by step answer:
Given data contains,
In FCC, the no. of atoms in per unit cell is 4. Z=4
M$ = 63.5g/mol$
a$ = 3.61 \times {10^{ - 8}}cm$
Avogadro’s number ${N_A} = 6.022 \times {10^{ - 23}}mo{l^{ - 1}}$
The arrangement of copper atoms shows a face-centered-cubic unit cell has four atoms per unit cell.
Let us calculate density by using the formula as,
$D = \dfrac{{ZM}}{{{a^3}{N_o}}}$
Substituting the given values in the formula we get,
$ \Rightarrow D = \dfrac{{4 \times 63.5g/mol}}{{{{\left( {3.61 \times {{10}^{ - 8}}cm} \right)}^3} \times 6.022 \times {{10}^{23}}mo{l^{ - 1}}}}$
$ \Rightarrow D = \dfrac{{254g/mol}}{{\left( {47.05 \times {{10}^{ - 24}}c{m^3}} \right) \times 6.022 \times {{10}^{23}}mol}}$
On simplifying we get,
$ \Rightarrow D = 8.97gc{m^{ - 3}}$
Finally, the density value is approximately equal to \[8.92{\text{ }}g{\text{ }}c{m^{ - 3}}\].
Note: Also we know that Primitive lattice or simple in which there are points at all the corners of the unit cell. The number of atoms per units cell in a primitive lattice is $8 \times (1/8) = 1$
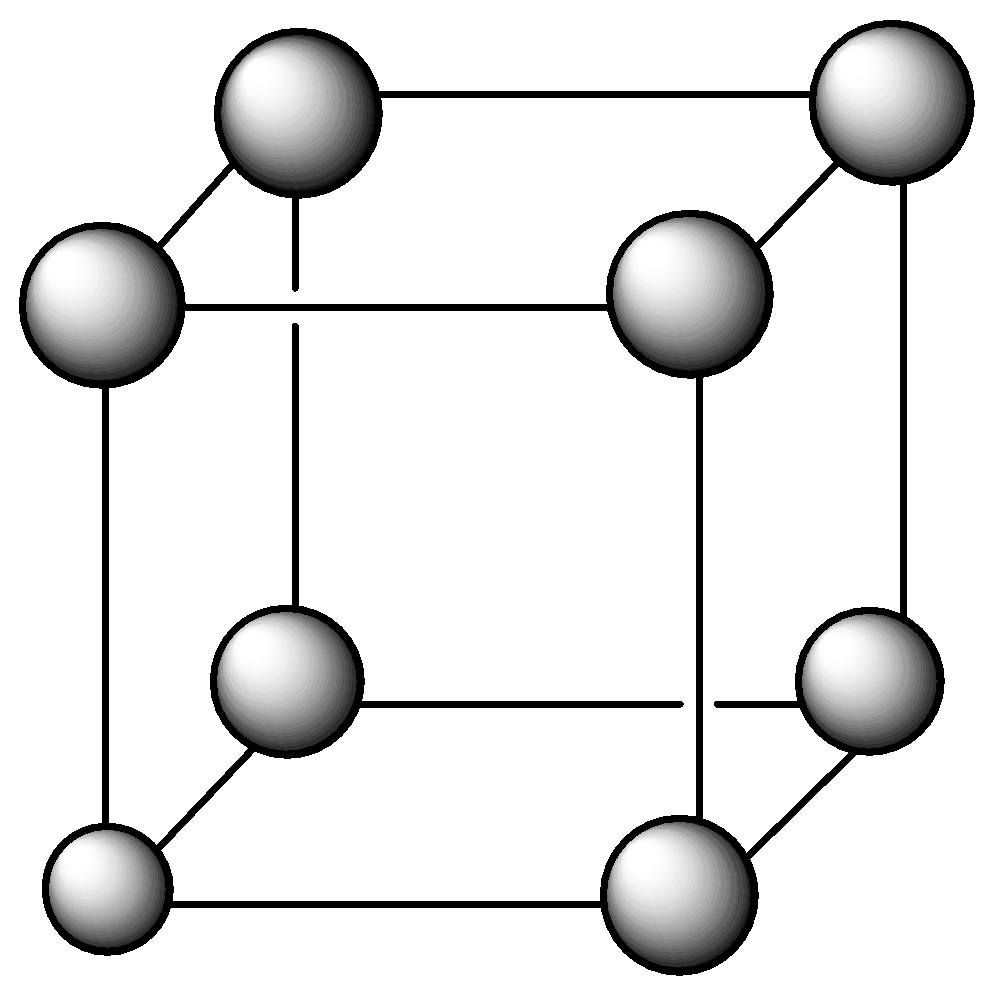
Body-centered lattice in which there are points at all corners as well as in the center of the unit cell. The number of atoms per unit cell is $8 \times (1/8) + 1 = 2$
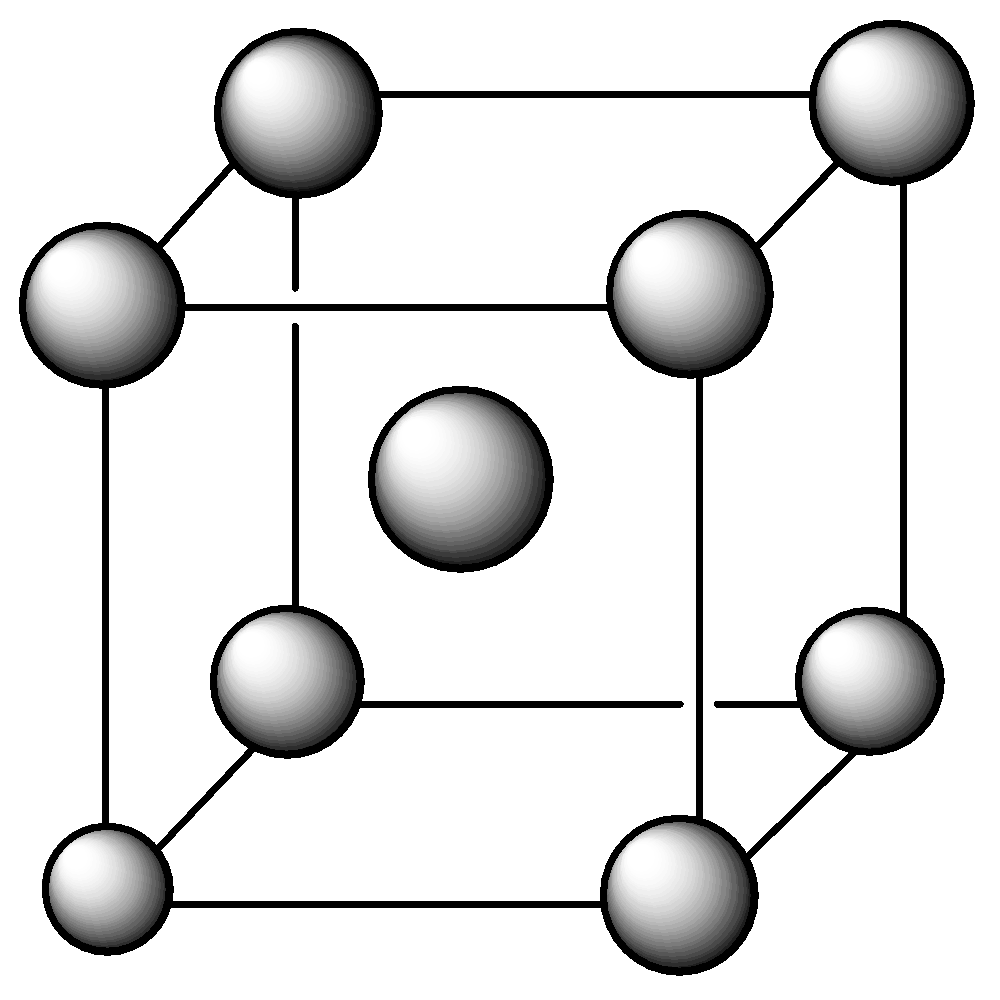
We must remember that an appointment of atoms in crystals during which the atomic centers are disposed in space is such that one atom is found at each of the corners of the cube and one at the middle of every face.
We know that in FCC, there are eight atoms at each corner (each shared by 8 unit cells) and six at the faces (each shared by two unit cells).
The number of atoms per unit cell in FCC is $8 \times (1/8) + 6 \times (1/2) = 4$ .
Formula Used:The formula of density is given as below,
$D = \dfrac{{ZM}}{{{a^3}{N_A}}}$
Where,
Z = No. of atoms in unit cell
M = Atomic mass of the substance
a = Edge length
$N_A$ = Avogadro number
Complete step by step answer:
Given data contains,
In FCC, the no. of atoms in per unit cell is 4. Z=4
M$ = 63.5g/mol$
a$ = 3.61 \times {10^{ - 8}}cm$
Avogadro’s number ${N_A} = 6.022 \times {10^{ - 23}}mo{l^{ - 1}}$
The arrangement of copper atoms shows a face-centered-cubic unit cell has four atoms per unit cell.
Let us calculate density by using the formula as,
$D = \dfrac{{ZM}}{{{a^3}{N_o}}}$
Substituting the given values in the formula we get,
$ \Rightarrow D = \dfrac{{4 \times 63.5g/mol}}{{{{\left( {3.61 \times {{10}^{ - 8}}cm} \right)}^3} \times 6.022 \times {{10}^{23}}mo{l^{ - 1}}}}$
$ \Rightarrow D = \dfrac{{254g/mol}}{{\left( {47.05 \times {{10}^{ - 24}}c{m^3}} \right) \times 6.022 \times {{10}^{23}}mol}}$
On simplifying we get,
$ \Rightarrow D = 8.97gc{m^{ - 3}}$
Finally, the density value is approximately equal to \[8.92{\text{ }}g{\text{ }}c{m^{ - 3}}\].
Note: Also we know that Primitive lattice or simple in which there are points at all the corners of the unit cell. The number of atoms per units cell in a primitive lattice is $8 \times (1/8) = 1$

Body-centered lattice in which there are points at all corners as well as in the center of the unit cell. The number of atoms per unit cell is $8 \times (1/8) + 1 = 2$

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




