
What is the correct formula of dinitrogen sulfide?
A) \[N{S_2}\]
B) \[N{S_{}}\]
C) \[{N_2}S\]
D) None of these
Answer
519.3k+ views
Hint: We have to remember that it is easy to identify the chemical formula by looking at the chemical name. For example: \[N\]can be written as nitrogen whereas \[{O_2}\] is Dioxide, So the formula for \[N{O_2}\] is nitrogen dioxide. Molecular weight of \[{N_2}S\] is $60g/mol$.
Complete answer:
It is easy to find the molecular formula, since it is dinitrogen then there must be two nitrogen atoms present in a molecule.
Option A) this is an incorrect option as \[N{S_2}\] represents disulfide instead of dinitrogen thus we can easily neglect this option.
Option B) This is an incorrect option as \[N{S_{}}\]does not represent dinitrogen sulfide as it does not have \[{N_2}\] in its formula.
Option C) this is a correct option as \[{N_2}S\] represents dinitrogen sulfide.
Dinitrogen sulfide has a structural formula
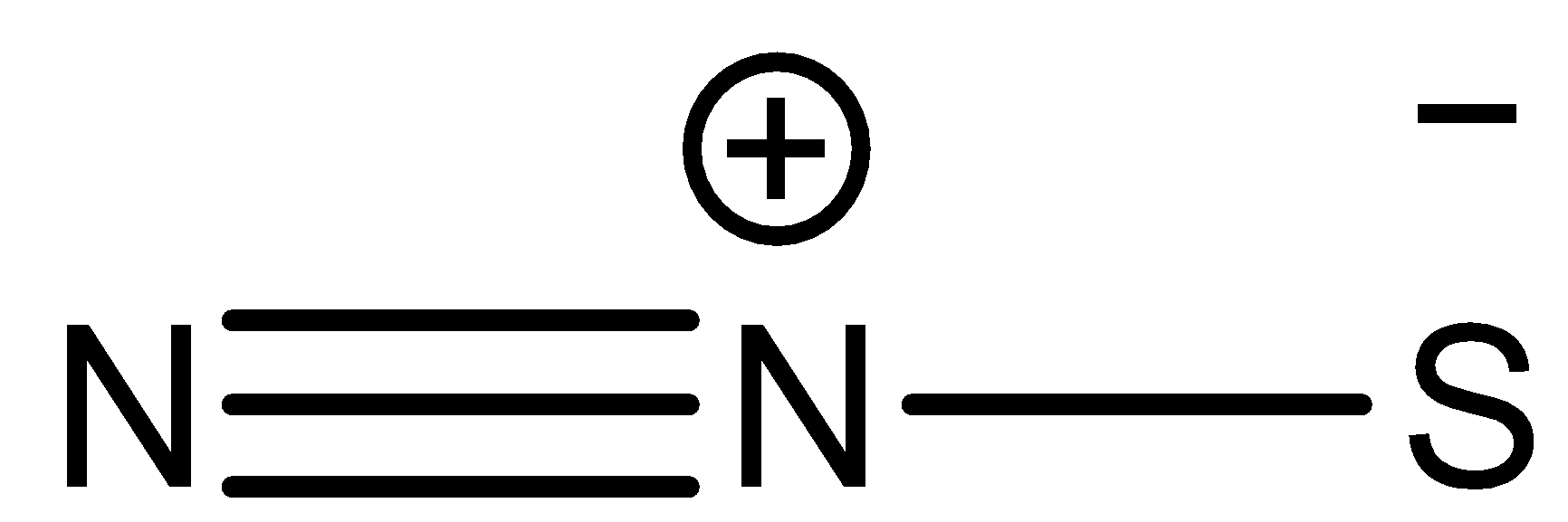
\[{N_2}S\] has a molecular weight$ = 60g/mol$. It is a highly polar molecule due to the presence of formal charges on it. It is a linear molecule which is considered to be stable and unreactive at room temperature.
Option D) this is an incorrect option as we got option C as a correct option.
Note:
We have to remember that the dinitrogen sulfide molecule is highly polar in nature since it has two opposite charges to attract between the bonds that hold an atom or a molecule. This molecule is a linear molecule that can be considered to be stable and unreactive at room temperature. In this molecule since sulphur is less electronegative than nitrogen thus electrons will prefer to lie on nitrogen atoms than sulphur.
Complete answer:
It is easy to find the molecular formula, since it is dinitrogen then there must be two nitrogen atoms present in a molecule.
Option A) this is an incorrect option as \[N{S_2}\] represents disulfide instead of dinitrogen thus we can easily neglect this option.
Option B) This is an incorrect option as \[N{S_{}}\]does not represent dinitrogen sulfide as it does not have \[{N_2}\] in its formula.
Option C) this is a correct option as \[{N_2}S\] represents dinitrogen sulfide.
Dinitrogen sulfide has a structural formula

\[{N_2}S\] has a molecular weight$ = 60g/mol$. It is a highly polar molecule due to the presence of formal charges on it. It is a linear molecule which is considered to be stable and unreactive at room temperature.
Option D) this is an incorrect option as we got option C as a correct option.
Note:
We have to remember that the dinitrogen sulfide molecule is highly polar in nature since it has two opposite charges to attract between the bonds that hold an atom or a molecule. This molecule is a linear molecule that can be considered to be stable and unreactive at room temperature. In this molecule since sulphur is less electronegative than nitrogen thus electrons will prefer to lie on nitrogen atoms than sulphur.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




