
What is the correct Lewis structure for arsenic?
Answer
478.5k+ views
Hint: Arsenic is the chemical element which belongs to the nitrogen family of the periodic table which is group $ 15 $ and the period of arsenic is $ 4 $ . Its atomic number is $ 33 $ . It is found in nature in the form of grey and yellow crystalline forms.
Complete answer:
Arsenic exists in nature in three allotropic forms which are black, yellow and grey in which a silver- grey are brittle crystalline solid and the stable forms. Arsenic oxidises rapidly when it is kept in contact with air and forms a white cloud of arsenic trioxide at high temperature.
Arsenic, like nitrogen, has five electrons in its valence shell. The position of arsenic is under the phosphorus which itself is under nitrogen, thus these elements are known as isoelectronic and they have similar properties.
Lewis structures are also termed as Lewis dot formulas are the diagrams which represent the bonding between the atoms present in the molecules also with lone pairs of electrons which may exist in the molecules.
Lewis structures are very useful for the summarization of the information about bonding and may be considered as “electron bookkeeping”. In Lewis dot structures, the electrons are represented by dots. And a pair of dots between symbols for atoms shows a bond.
The Lewis dot structure of Arsenic is as follows:
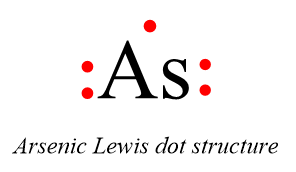
Thus this is the required answer.
Note:
In inorganic form, Arsenic is extremely dangerous. The most harmful effects shown by arsenic to human health is cancer and skin sores. Arsenic poisoning is from drinking contaminated water, eating food prepared by using them and irrigation of food crops.
Complete answer:
Arsenic exists in nature in three allotropic forms which are black, yellow and grey in which a silver- grey are brittle crystalline solid and the stable forms. Arsenic oxidises rapidly when it is kept in contact with air and forms a white cloud of arsenic trioxide at high temperature.
Arsenic, like nitrogen, has five electrons in its valence shell. The position of arsenic is under the phosphorus which itself is under nitrogen, thus these elements are known as isoelectronic and they have similar properties.
Lewis structures are also termed as Lewis dot formulas are the diagrams which represent the bonding between the atoms present in the molecules also with lone pairs of electrons which may exist in the molecules.
Lewis structures are very useful for the summarization of the information about bonding and may be considered as “electron bookkeeping”. In Lewis dot structures, the electrons are represented by dots. And a pair of dots between symbols for atoms shows a bond.
The Lewis dot structure of Arsenic is as follows:

Thus this is the required answer.
Note:
In inorganic form, Arsenic is extremely dangerous. The most harmful effects shown by arsenic to human health is cancer and skin sores. Arsenic poisoning is from drinking contaminated water, eating food prepared by using them and irrigation of food crops.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




