
What is the correct name of $I{{F}_{7}}$.
(A) Iron heptafluoride.
(B) Iodine heptafluoride
(C) Iodine septafluroide
(D) Iron (IV) septafluoride.
Answer
585.9k+ views
Hint: $I{{F}_{7}}$ is the best example for interhalogen compounds. Interhalogen compounds mean halogens themselves react to each other and form interhalogen compounds. $I{{F}_{7}}$ has a structure of pentagonal bipyramidal.
Complete step by step solution:
-In $I{{F}_{7}}$, one iodine and seven fluorine atoms are present.
-The chemical name of $I{{F}_{7}}$ is Iodine heptafluoride.
-In the chemical name of $I{{F}_{7}}$ the name of Iodine should be written first because of its less electronegativity. Fluorine's name should be written later because it is more electronegativity.
-The prefix hepta in the name of $I{{F}_{7}}$ - Iodine heptafluoride indicates the presence of seven fluorine atoms.
So, the correct option is (B).
Additional information:
-The structure of $I{{F}_{7}}$ is as follows.
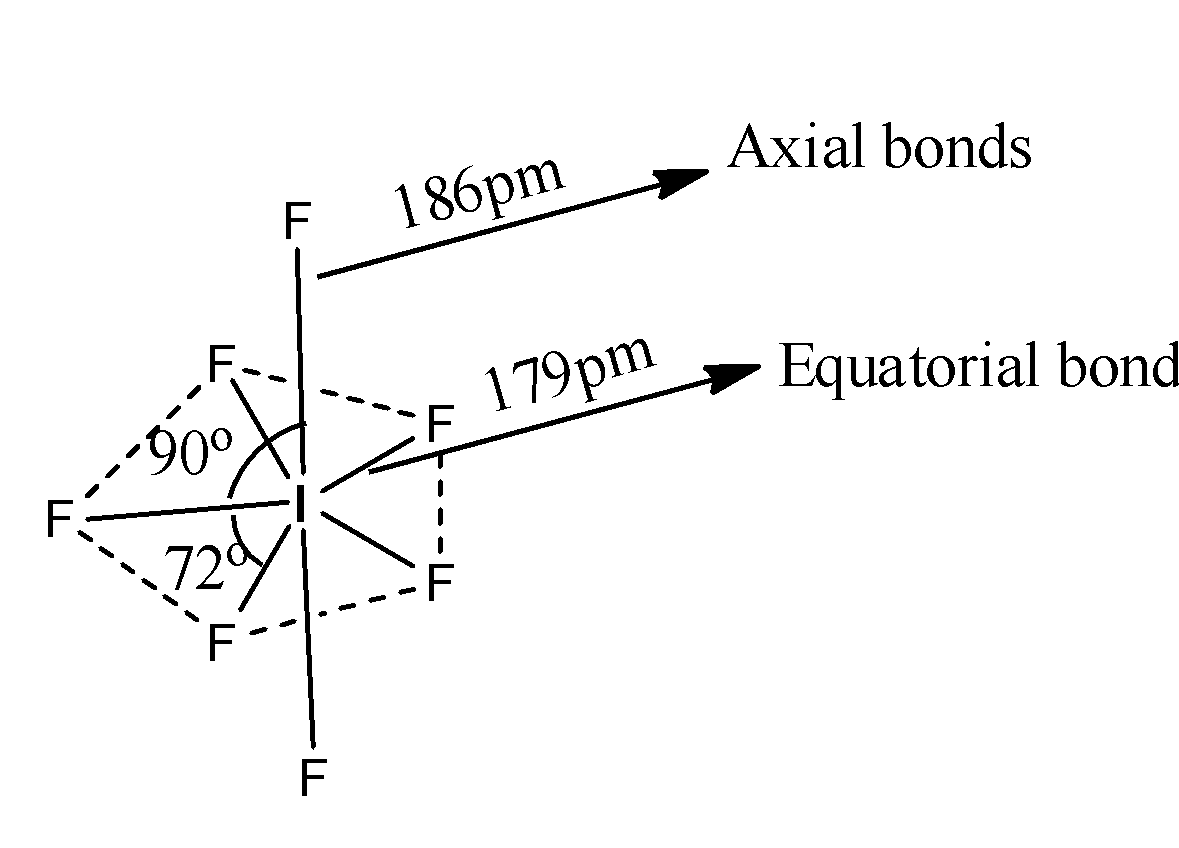
-The resultant geometry of $I{{F}_{7}}$ is pentagonal bipyramidal.
-The hybridization of Iodine in $I{{F}_{7}}$ is $s{{p}^{3}}{{d}^{3}}$ and the overlapping of fluorine with iodine orbitals we can see as follows.
-There are two types of bonds axial and equatorial bonds.
-Axial bonds are longer than equatorial bonds.
-There are two types of orthogonal angles, there are 90 and 72.
Note: If any chemical contains a number of repeating atoms in its chemical formula then we are not supposed to write the number in the chemical name. If the compound contains Two same atoms – di, three same atoms – tri, four same atoms – tetra, five same atoms – penta, six same atoms – hexa, seven same atoms – septa, eight same atoms – octa.
Complete step by step solution:
-In $I{{F}_{7}}$, one iodine and seven fluorine atoms are present.
-The chemical name of $I{{F}_{7}}$ is Iodine heptafluoride.
-In the chemical name of $I{{F}_{7}}$ the name of Iodine should be written first because of its less electronegativity. Fluorine's name should be written later because it is more electronegativity.
-The prefix hepta in the name of $I{{F}_{7}}$ - Iodine heptafluoride indicates the presence of seven fluorine atoms.
So, the correct option is (B).
Additional information:
-The structure of $I{{F}_{7}}$ is as follows.

-The resultant geometry of $I{{F}_{7}}$ is pentagonal bipyramidal.
-The hybridization of Iodine in $I{{F}_{7}}$ is $s{{p}^{3}}{{d}^{3}}$ and the overlapping of fluorine with iodine orbitals we can see as follows.
-There are two types of bonds axial and equatorial bonds.
-Axial bonds are longer than equatorial bonds.
-There are two types of orthogonal angles, there are 90 and 72.
Note: If any chemical contains a number of repeating atoms in its chemical formula then we are not supposed to write the number in the chemical name. If the compound contains Two same atoms – di, three same atoms – tri, four same atoms – tetra, five same atoms – penta, six same atoms – hexa, seven same atoms – septa, eight same atoms – octa.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




