
How many covalent bonds are present in pentane ${C_5}{H_{12}}$ ?
Answer
607.2k+ views
Hint: - For answering these types of questions we must understand the atomic structure of Carbon and Hydrogen and also the molecular structure of ${C_5}{H_{12}}$.
Complete step-by-step answer:
A covalent bond, also called a molecular bond, is a chemical bond which involves the sharing of pairs of electrons among atoms. These electron pairs are known as joint pairs or bonding pairs, and when they share electrons, the stable equilibrium of attractive and repulsive forces between atoms is known as covalent bonding.
Carbon's atomic number is 6, and the atomic mass number is 12. This has 6 protons, 6 neutrons and 6 electrons and there are 4 electrons in the valence shell.
A hydrogen atom is an electrically neutral atom containing a single proton charged positively, and a single electron charged negatively.
Molecular structure of pentane is ${C_5}{H_{12}}$
The formula is given by structure: -
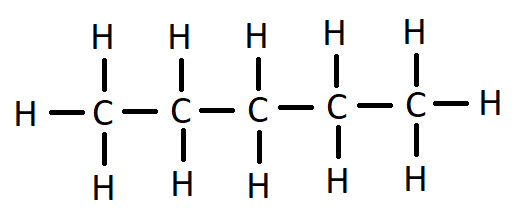
Here the number of $C - C$ covalent bonds is 4 and the number of $C - H$ covalent bonds are 12
So, the total number of covalent bonds is = 12+4 =16
Note: - The general formulation is that in theory, the nth shell will accommodate up to $2({n^2})$ electrons where n is the number of shells. So, there will be 8 electrons in the outermost shell of Carbon and 2 electrons in hydrogen. Hence, Carbon and Hydrogen pair with each other to fulfil their requirement in the given arrangement above.
Complete step-by-step answer:
A covalent bond, also called a molecular bond, is a chemical bond which involves the sharing of pairs of electrons among atoms. These electron pairs are known as joint pairs or bonding pairs, and when they share electrons, the stable equilibrium of attractive and repulsive forces between atoms is known as covalent bonding.
Carbon's atomic number is 6, and the atomic mass number is 12. This has 6 protons, 6 neutrons and 6 electrons and there are 4 electrons in the valence shell.
A hydrogen atom is an electrically neutral atom containing a single proton charged positively, and a single electron charged negatively.
Molecular structure of pentane is ${C_5}{H_{12}}$
The formula is given by structure: -
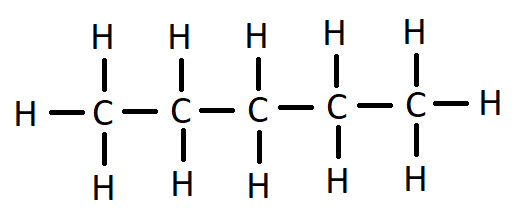
Here the number of $C - C$ covalent bonds is 4 and the number of $C - H$ covalent bonds are 12
So, the total number of covalent bonds is = 12+4 =16
Note: - The general formulation is that in theory, the nth shell will accommodate up to $2({n^2})$ electrons where n is the number of shells. So, there will be 8 electrons in the outermost shell of Carbon and 2 electrons in hydrogen. Hence, Carbon and Hydrogen pair with each other to fulfil their requirement in the given arrangement above.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




