
How many cyclic structures are possible for \[{C_4}{H_6}\]?
Answer
506.7k+ views
Hint: As we know that the full form of IUPAC is the International Union of Pure and Applied Chemistry. It is a World Wide organisation for the nomenclature of the new elements in the periodic table and chemical molecules. The IUPAC has certain rules and regulations for the naming of the organic compound. In that method only we named the organic molecules in World level. The naming of any organic molecule depends on the parent saturated hydrocarbon. In organic chemistry, hydrocarbons are an important topic. The hydrocarbons are majorly classified as three groups. There are alkane, alkene and alkyne. The alkane means carbon-carbon single bond. The alkene has a carbon-carbon double bond. The alkyne means carbon-carbon having triple bond in the molecule. It has some general formulas. The general formula of alkane is \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n + 2}}}}\]. The general formula of alkene is \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n}}}}\]. The general formula of alkyne is \[{{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n - 2}}}}\].
Complete answer:
The given molecular formula is \[{C_4}{H_6}\].
The possible structure of the give molecular formula is given below,
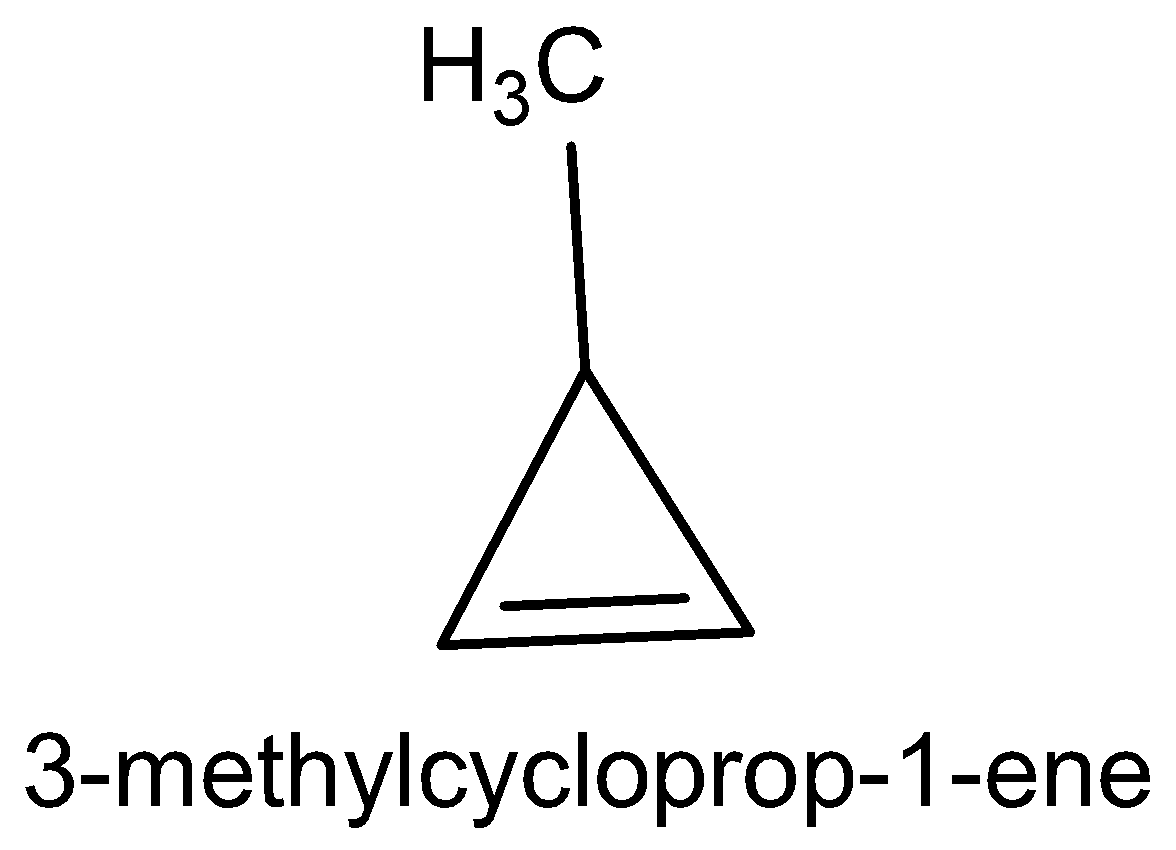
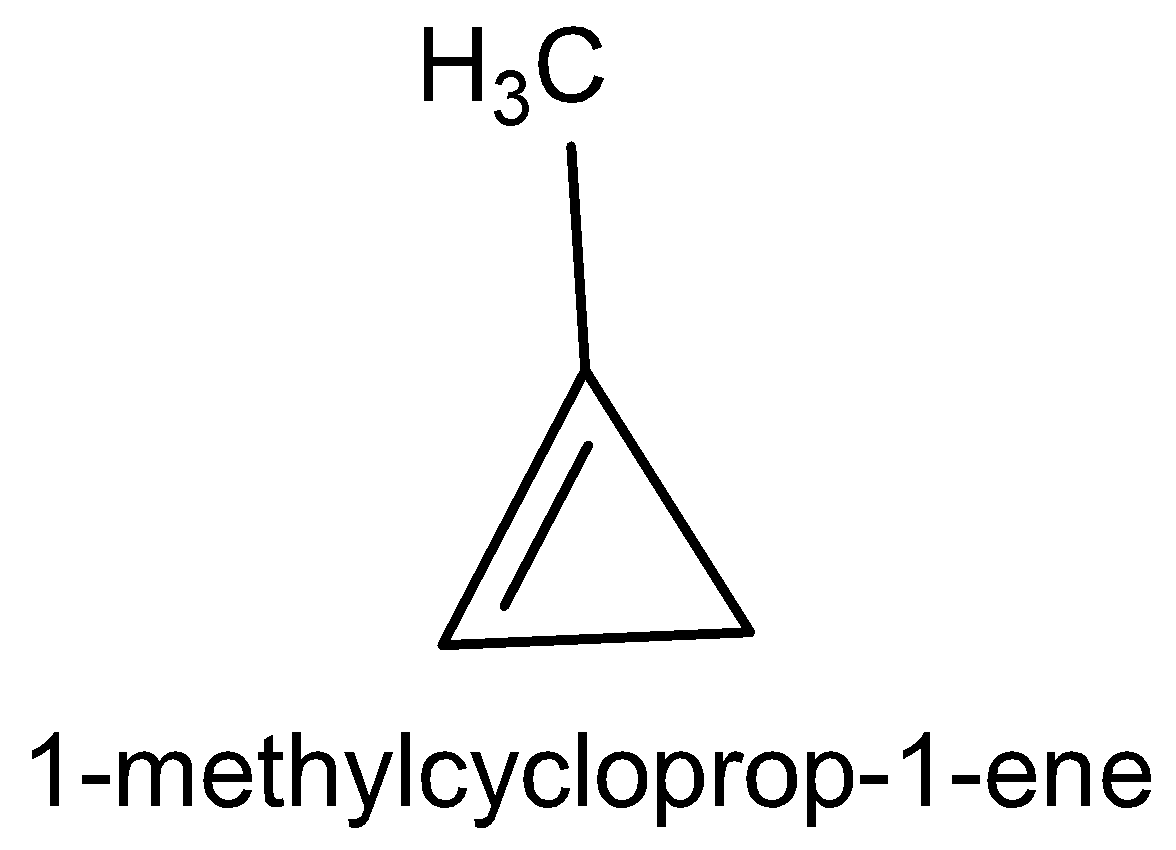
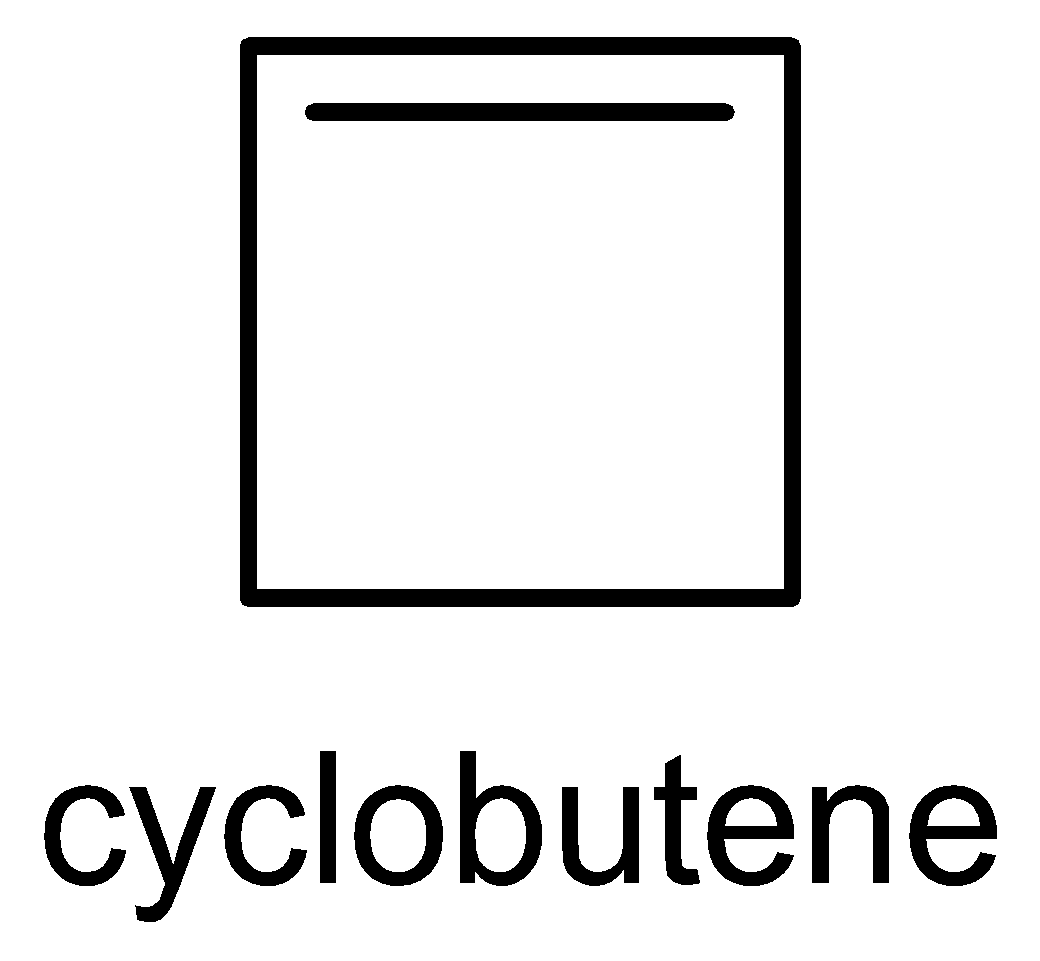
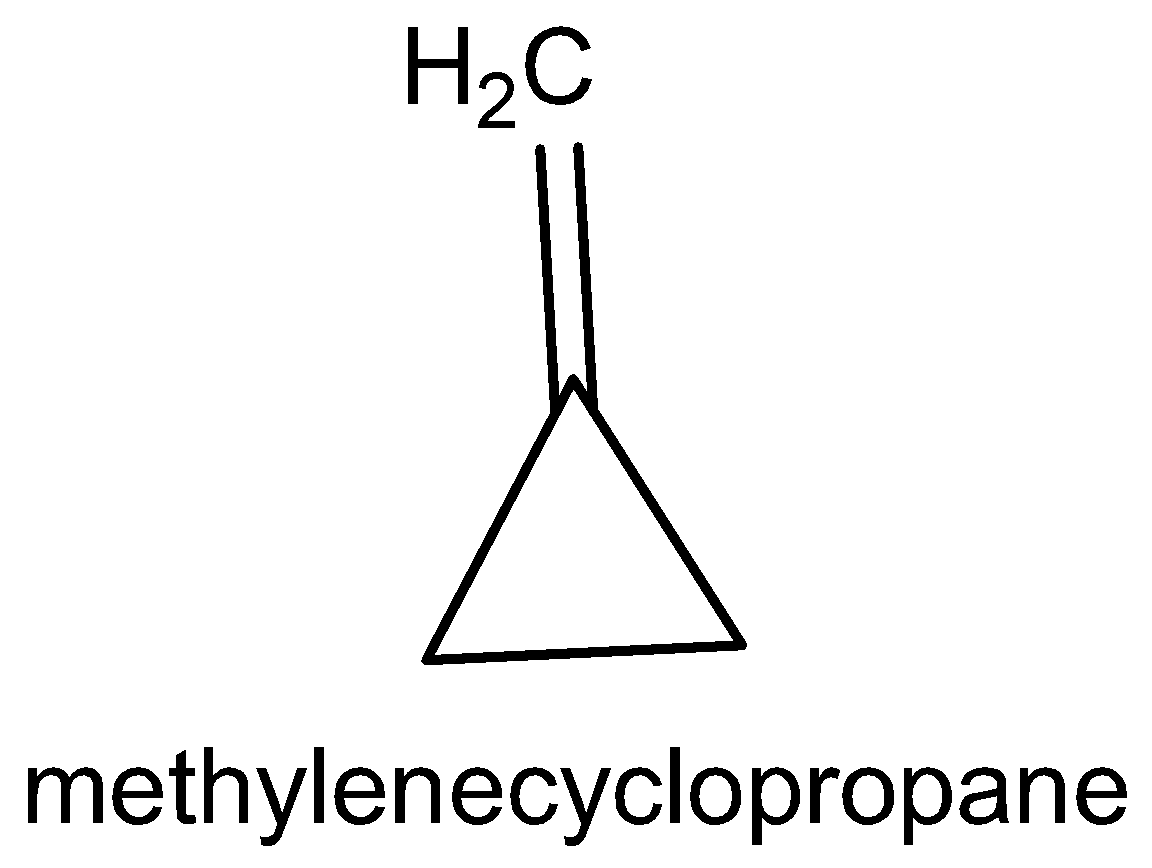
The five cyclic structure names are methylene cyclopropane, cyclobutane, 3-methylcyclo propane, 1-methylcyclo propane and bicycle butane.
According to the above discussion, we conclude that there are five cyclic structures possible in the given molecular formula \[{C_4}{H_6}\].
Note:
We need to know that the functional group of the molecule is very important for the naming time. If one molecule having more than one functional group means some priority list is also provided from IUPAC. If the number of substituent and functional group is dependent on the minimum number will come from the left side or right of the terminal carbon atom in the chain. The aldehyde functional group in the molecule ends with al. it is one of the IUPAC rules for the naming of functional groups.
Complete answer:
The given molecular formula is \[{C_4}{H_6}\].
The possible structure of the give molecular formula is given below,
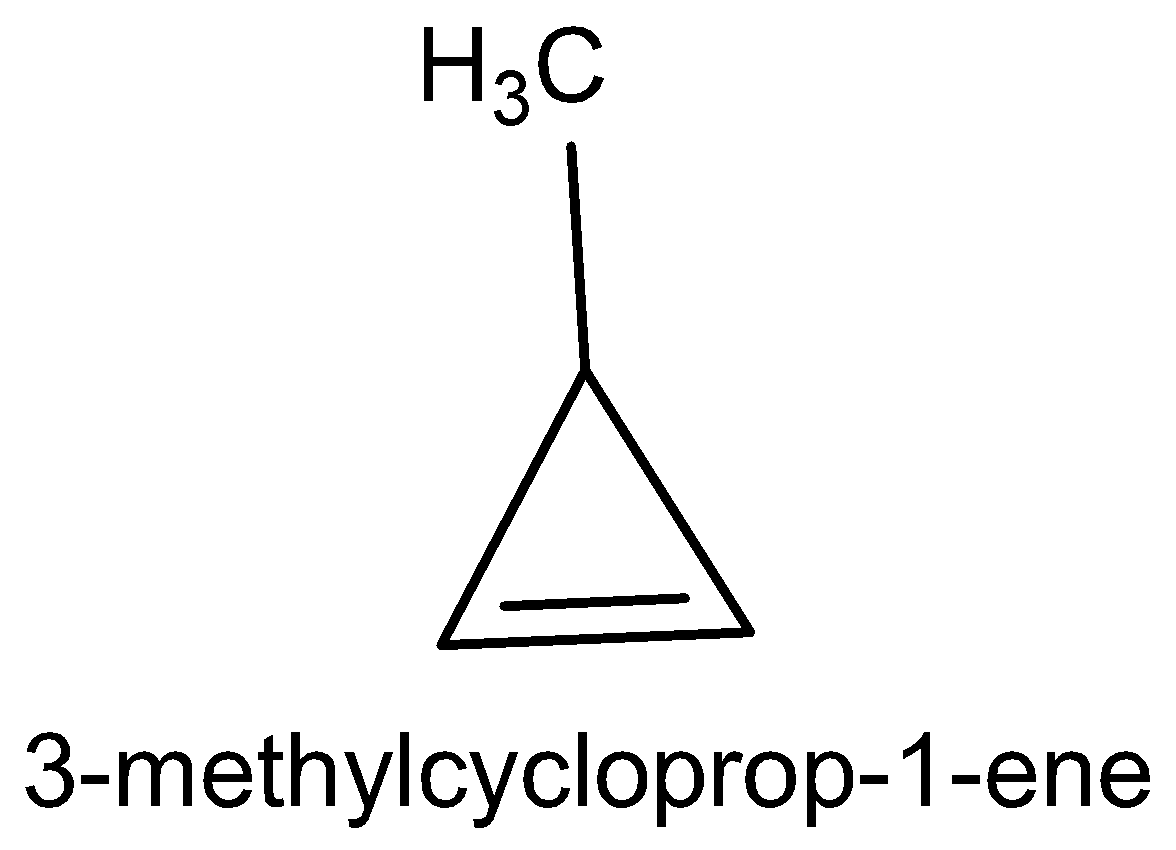
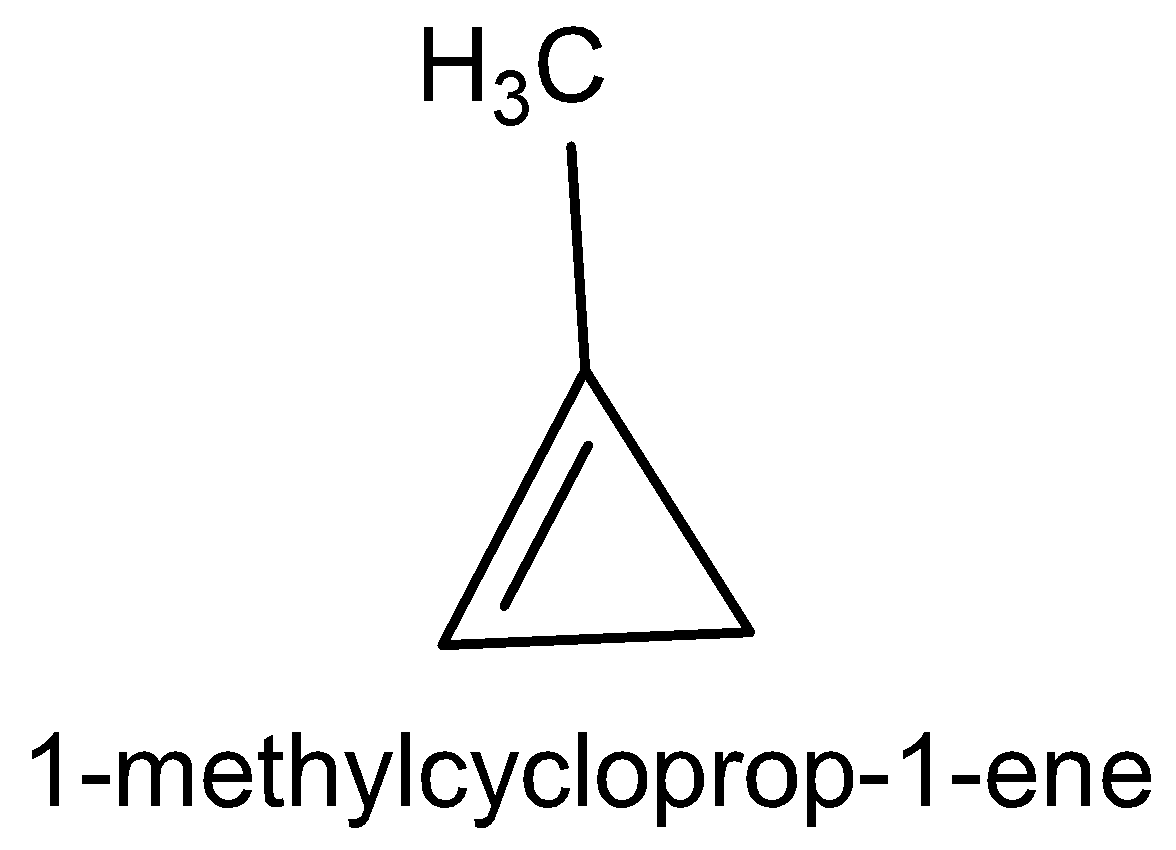



The five cyclic structure names are methylene cyclopropane, cyclobutane, 3-methylcyclo propane, 1-methylcyclo propane and bicycle butane.
According to the above discussion, we conclude that there are five cyclic structures possible in the given molecular formula \[{C_4}{H_6}\].
Note:
We need to know that the functional group of the molecule is very important for the naming time. If one molecule having more than one functional group means some priority list is also provided from IUPAC. If the number of substituent and functional group is dependent on the minimum number will come from the left side or right of the terminal carbon atom in the chain. The aldehyde functional group in the molecule ends with al. it is one of the IUPAC rules for the naming of functional groups.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




