
Dacron is obtained by the condensation polymerization of:
(A) dimethyl terephthalate and ethylene glycol
(B) terephthalic acid and ethylene glycol
(C) phenol and phthalic acid
(D) phenol and formaldehyde
Answer
586.2k+ views
Hint: Polymers are macromolecules built up by linking together a large number of small molecules. The repeating units in a polymer are linked through strong covalent bonds. The process of combining small molecules to form polymer molecules is called polymerization. Three types of polymerization classified based on the properties of monomers are addition polymerization, condensation polymerization, and co-polymerization.
Complete step by step solution:
There are three types of synthesis based on the monomers combine to form polymer:
(1) Addition polymerization, examples: PE, PVC, PTFE, etc.
(2) Condensation polymerization, Examples: PET, Bakelite, Dacron, etc.
(3) Co-polymerization, examples: BuNa-S and BuNa-N
Condensation polymerization: the synthesis of two or more different monomers with functional groups are combined to form polymers by releasing small products like water, ammonia, etc. is known as condensation polymerization or step-growth polymerization. The polymer is formed in this process named as condensation polymer.
Example: condensation polymerization of Dacron
Dacron is a polymer, which is formed from the condensation polymerization of ethylene glycol and terephthalic acid.
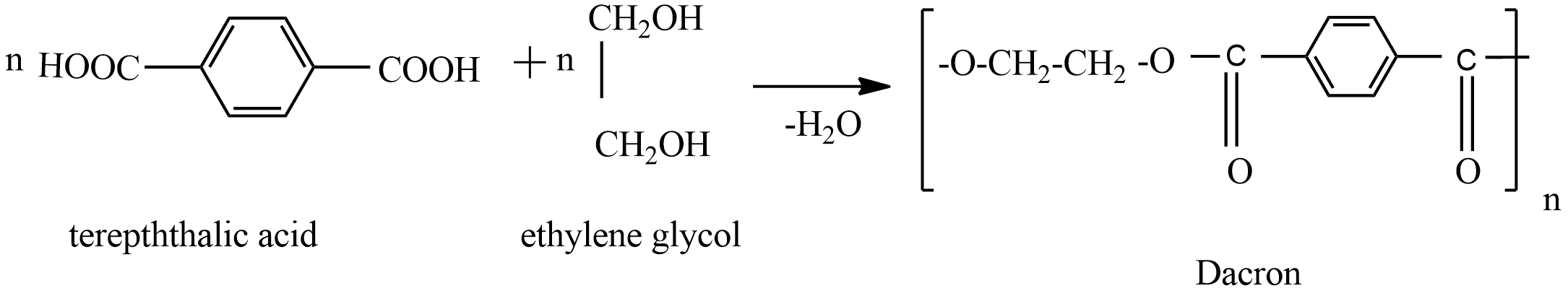
Hence, Dacron is obtained by the condensation polymerization of terephthalic acid and ethylene glycol.
So, the correct answer is option B.
Note: Dacron is a type of synthetic rubber and is also known as Terylene. This is a form of polyester which is formed with ester groups attached to the main chain. This is a high tensile strength and high resistance material with good resistance to chemical degradation by chemical bleaches.
Complete step by step solution:
There are three types of synthesis based on the monomers combine to form polymer:
(1) Addition polymerization, examples: PE, PVC, PTFE, etc.
(2) Condensation polymerization, Examples: PET, Bakelite, Dacron, etc.
(3) Co-polymerization, examples: BuNa-S and BuNa-N
Condensation polymerization: the synthesis of two or more different monomers with functional groups are combined to form polymers by releasing small products like water, ammonia, etc. is known as condensation polymerization or step-growth polymerization. The polymer is formed in this process named as condensation polymer.
Example: condensation polymerization of Dacron
Dacron is a polymer, which is formed from the condensation polymerization of ethylene glycol and terephthalic acid.

Hence, Dacron is obtained by the condensation polymerization of terephthalic acid and ethylene glycol.
So, the correct answer is option B.
Note: Dacron is a type of synthetic rubber and is also known as Terylene. This is a form of polyester which is formed with ester groups attached to the main chain. This is a high tensile strength and high resistance material with good resistance to chemical degradation by chemical bleaches.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




