
Why is damage to the ozone layer a cause for concern? What steps are being taken to limit this damage?
Answer
579k+ views
Hint: It is a layer that is present at the stratosphere layer of the earth at about 10 km of an altitude containing a high concentration of ozone that helps in absorbing most of the ultraviolet rays that reach the earth from the sun.
Complete step by step answer:
The layer of ozone gas within the upper atmosphere is understood as the ozonosphere. The Ozone layer is vital for the existence of life on earth as it absorbs most of the harmful ultraviolet radiation coming from the sun and prevents them from reaching the earth and protects from damages to our body.
Ultraviolet rays can cause various damages to our body like:
- Skin cancer
- Serious eye disease is known as a cataract.
- damaging our immune systems
- Damage to the agriculture that is to the crops.
The steps being taken to limit the damages of ultraviolet rays are the following:
- Limiting the use of substances that lead to ozone depletions such as Cfc which are produced from electrical appliances like Refrigerator and the manufactures of these appliances were requested to minimize the use of CFC (Chlorofluorocarbon) and to make eco- friendly products.
Additional Information: An oxygen molecule and an oxygen atom in the atmosphere join together to form ozone($O_3$). And so the combination of an ozone atom with the free oxygen atom leads to the formation of 2 oxygen molecules. See the illustrated chemical equation for more clarification.
${ O }+{ O }_{ 3 }\xrightarrow [ \quad ]{ \quad } { 2 }{ O }_{ 2 }$
The total amount of ozone formed in the stratosphere can be determined by the balance between photochemical production and recombination, both.
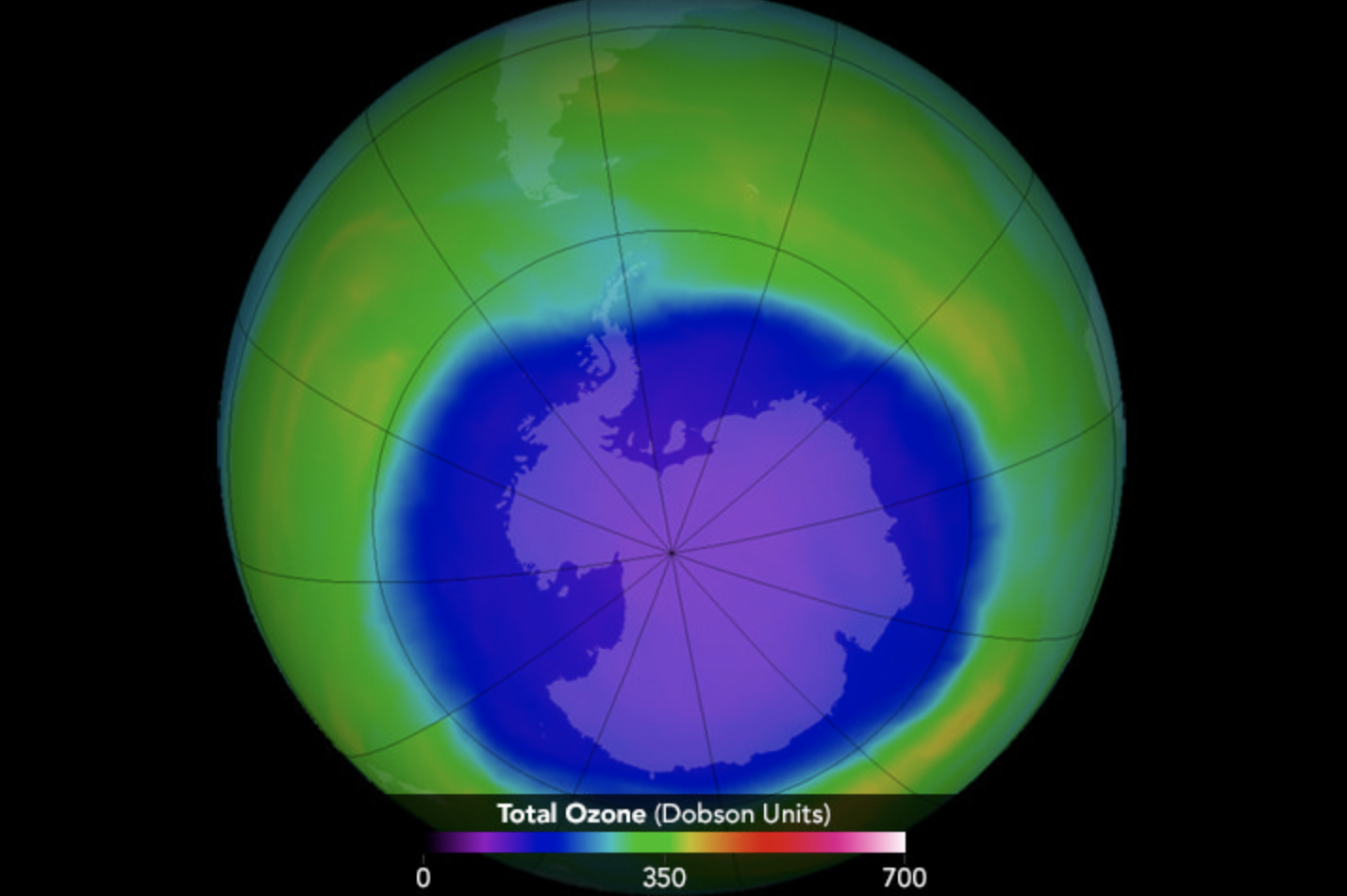
Note: Ozone depletion consists mainly of two related events which have been observed since the late 1970s: that's a gentle lowering of about four percent within the total amount of ozone in Earth's atmosphere (the ozone layer) and away from larger springtime decrease in stratospheric ozone around Earth's polar regions. The latter phenomenon is mentioned because of the hole.
Complete step by step answer:
The layer of ozone gas within the upper atmosphere is understood as the ozonosphere. The Ozone layer is vital for the existence of life on earth as it absorbs most of the harmful ultraviolet radiation coming from the sun and prevents them from reaching the earth and protects from damages to our body.
Ultraviolet rays can cause various damages to our body like:
- Skin cancer
- Serious eye disease is known as a cataract.
- damaging our immune systems
- Damage to the agriculture that is to the crops.

The steps being taken to limit the damages of ultraviolet rays are the following:
- Limiting the use of substances that lead to ozone depletions such as Cfc which are produced from electrical appliances like Refrigerator and the manufactures of these appliances were requested to minimize the use of CFC (Chlorofluorocarbon) and to make eco- friendly products.
Additional Information: An oxygen molecule and an oxygen atom in the atmosphere join together to form ozone($O_3$). And so the combination of an ozone atom with the free oxygen atom leads to the formation of 2 oxygen molecules. See the illustrated chemical equation for more clarification.
${ O }+{ O }_{ 3 }\xrightarrow [ \quad ]{ \quad } { 2 }{ O }_{ 2 }$
The total amount of ozone formed in the stratosphere can be determined by the balance between photochemical production and recombination, both.
Note: Ozone depletion consists mainly of two related events which have been observed since the late 1970s: that's a gentle lowering of about four percent within the total amount of ozone in Earth's atmosphere (the ozone layer) and away from larger springtime decrease in stratospheric ozone around Earth's polar regions. The latter phenomenon is mentioned because of the hole.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




