
Dative bond is present in
A. carbon monoxide
B. carbon dioxide
C. nitric oxide
D. Dichlorine monoxide
Answer
578.1k+ views
Hint: A bond is formed between two atoms. Both the atoms donate an electron to form the bond so, an electron pair is shared between the two bonded atoms. This bond is known as a covalent bond. The molecule in which one atom donates an electron pair to another atom to form the bond is known as a dative bond.
Complete answer:
When both the electrons of the shared pair are donated by one atom and shared between both of the bonded atoms is known as a dative bond. A dative bond is also known as a coordinate bond or dipolar bond.
We can draw the structures of the molecules to determine the dative bonds.
The structure of all the molecules is as follows:
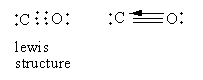
In carbon monoxide, two bonds are formed by the sharing of two electrons from each carbon and oxygen. The third bond is formed by the electron pair donated by the oxygen atom so carbon monoxide has a dative bond. So, option (A) is correct.
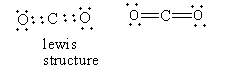
In carbon dioxide, four carbon-oxygen bonds are present. Each bond is formed by the donation of an electron from each atom so carbon dioxide does not dative bond so option (B) is incorrect.
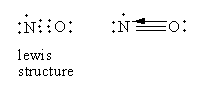
In nitric oxide, two bonds are formed by the sharing of two electrons from each, nitrogen, and oxygen. The third bond is formed by the electron pair donated by the oxygen atom so nitric oxide has a dative bond. So, option (C) is correct.
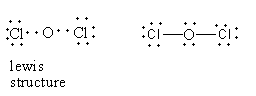
In dichlorine monoxide, two chlorine-oxygen bonds are present. Each bond is formed by the donation of an electron from each atom so dichlorine monoxide does not dative bond, so option (D) is incorrect.
Therefore, option (A) carbon monoxide and option (C) nitric oxide, are correct.
Note: In ionic bonding also one atom donates an electron to another but it is different from dative bonding because in dative bonding the atoms are not charged whereas in ionic bonding the electron pair is donated from anion to cation.
Complete answer:
When both the electrons of the shared pair are donated by one atom and shared between both of the bonded atoms is known as a dative bond. A dative bond is also known as a coordinate bond or dipolar bond.
We can draw the structures of the molecules to determine the dative bonds.
The structure of all the molecules is as follows:
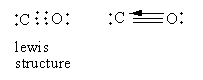
In carbon monoxide, two bonds are formed by the sharing of two electrons from each carbon and oxygen. The third bond is formed by the electron pair donated by the oxygen atom so carbon monoxide has a dative bond. So, option (A) is correct.
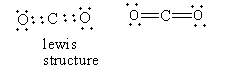
In carbon dioxide, four carbon-oxygen bonds are present. Each bond is formed by the donation of an electron from each atom so carbon dioxide does not dative bond so option (B) is incorrect.
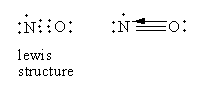
In nitric oxide, two bonds are formed by the sharing of two electrons from each, nitrogen, and oxygen. The third bond is formed by the electron pair donated by the oxygen atom so nitric oxide has a dative bond. So, option (C) is correct.
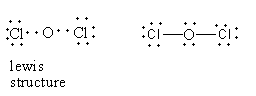
In dichlorine monoxide, two chlorine-oxygen bonds are present. Each bond is formed by the donation of an electron from each atom so dichlorine monoxide does not dative bond, so option (D) is incorrect.
Therefore, option (A) carbon monoxide and option (C) nitric oxide, are correct.
Note: In ionic bonding also one atom donates an electron to another but it is different from dative bonding because in dative bonding the atoms are not charged whereas in ionic bonding the electron pair is donated from anion to cation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




