
What is the decreasing order of bond angles in $B{{F}_{3}}$, $N{{H}_{3}}$, $P{{F}_{3}}$, and ${{I}_{3}}^{-}$ ?
A. $B{{F}_{3}}$ $>$ ${{I}_{3}}^{-}$ $>$ $P{{F}_{3}}$ $>$ $N{{H}_{3}}$
B. ${{I}_{3}}^{-}$ $>$ $N{{H}_{3}}$ $>$ $P{{F}_{3}}$ $>$ $B{{F}_{3}}$
C. $B{{F}_{3}}$ $>$ $N{{H}_{3}}$ $>$ $P{{F}_{3}}$ $>$ ${{I}_{3}}^{-}$
D. ${{I}_{3}}^{-}$ $>$ $B{{F}_{3}}$ $>$ $N{{H}_{3}}$ $>$ $P{{F}_{3}}$
Answer
592.2k+ views
Hint: Think about the hybridization, 3 dimensional orientation, and number of lone pairs on each central atom to identify the basic bond angle of the structure and then move on to how the lone pairs will affect the bond angle.
Complete step by step answer:
First, let us look at the geometry of all the molecules:
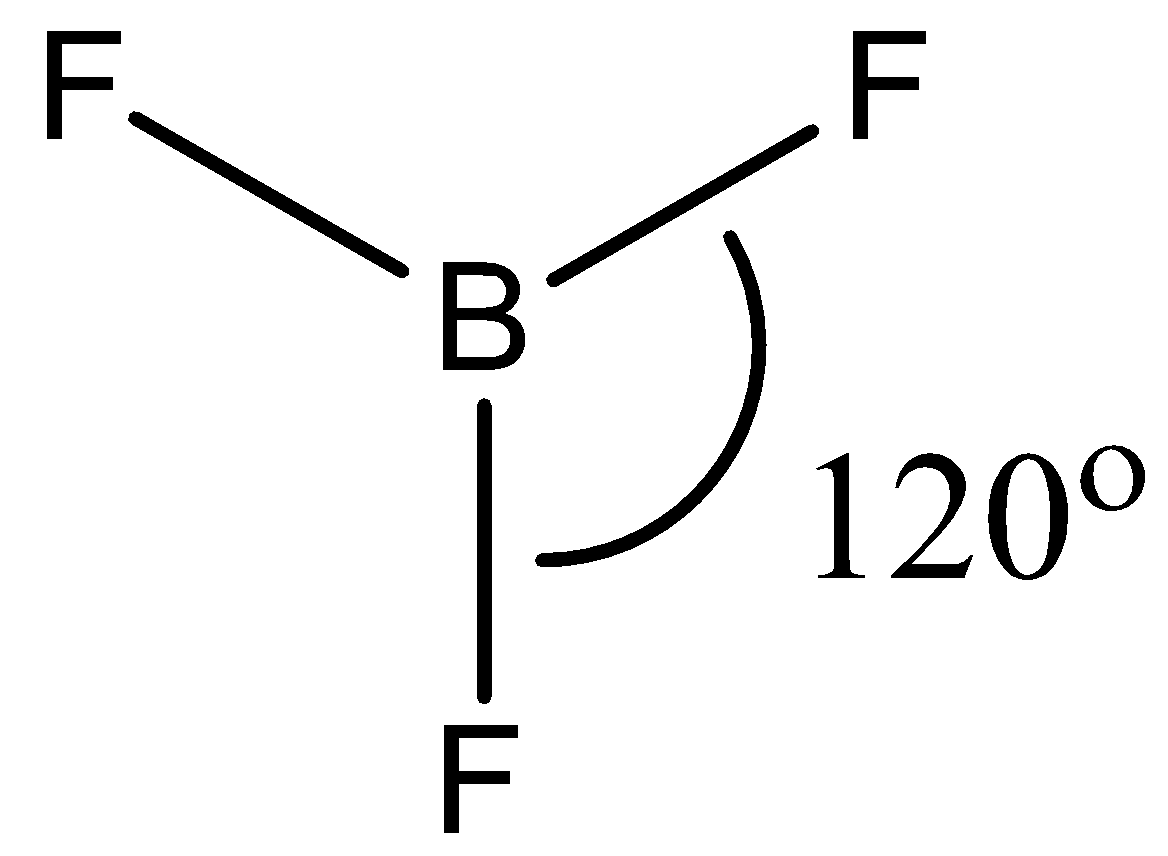
- The geometry of $B{{F}_{3}}$ is trigonal planar. It involves 4 atoms, no lone pairs with a hybridization of $s{{p}^{2}}$. This means that the bond angle between the atoms will be $120{}^\circ $.
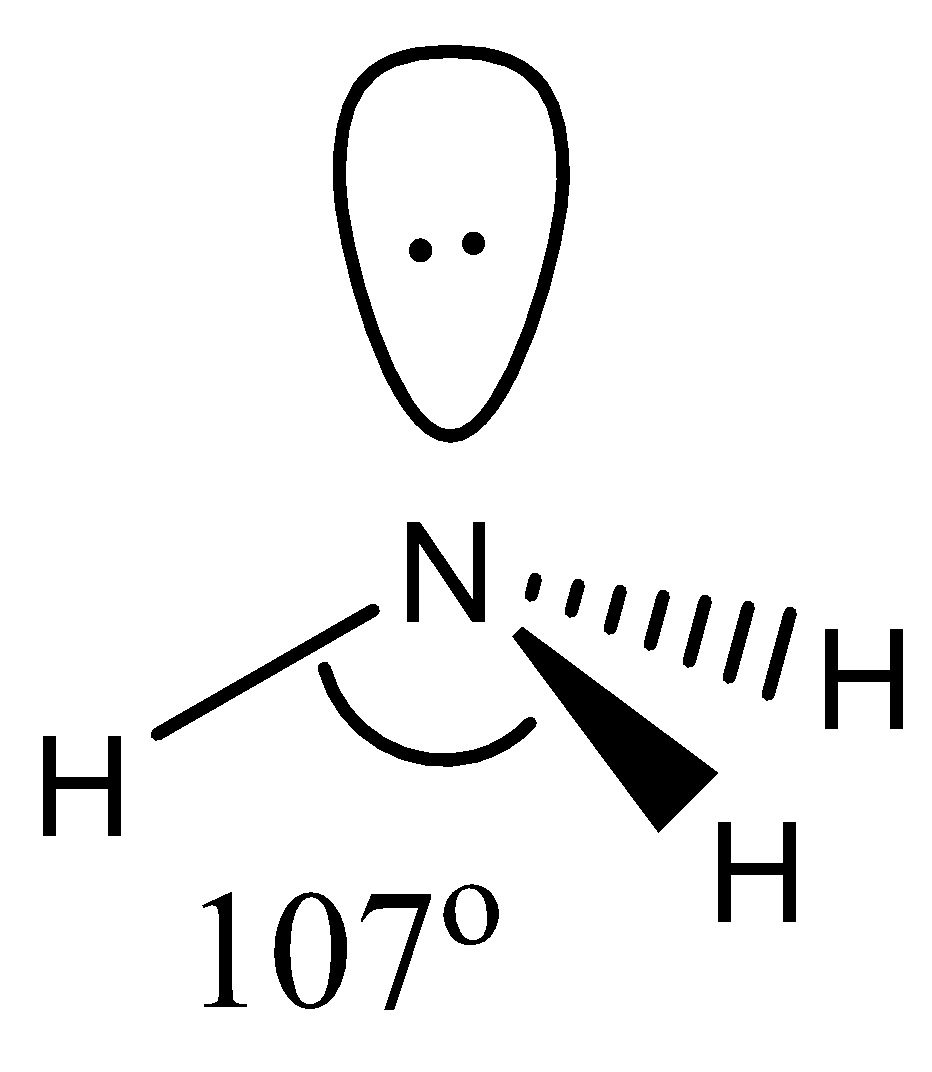
- The geometry of $N{{H}_{3}}$ is pyramidal. Technically, the geometry is tetrahedral, but since one of the corners is occupied by a lone pair it is sometimes called pyramidal. It involves 4 atoms, 1 lone pair with a hybridization of $s{{p}^{3}}$. This means that the bond angle will be less than $109{}^\circ $ due to the presence of a lone pair. It is found to be around $107{}^\circ $.
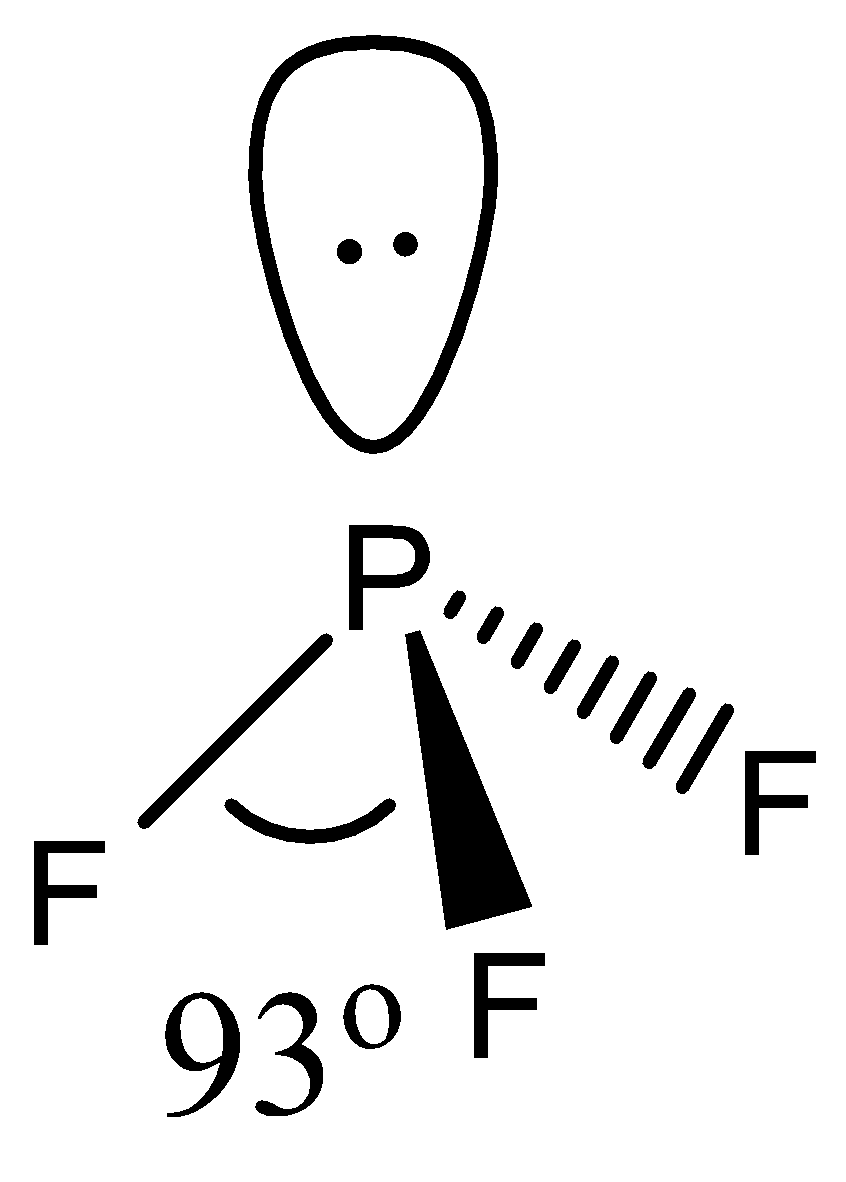
- The geometry of $P{{F}_{3}}$ is again tetrahedral. It involves 4 atoms, 1 lone pair with a hybridization of $s{{p}^{3}}$.
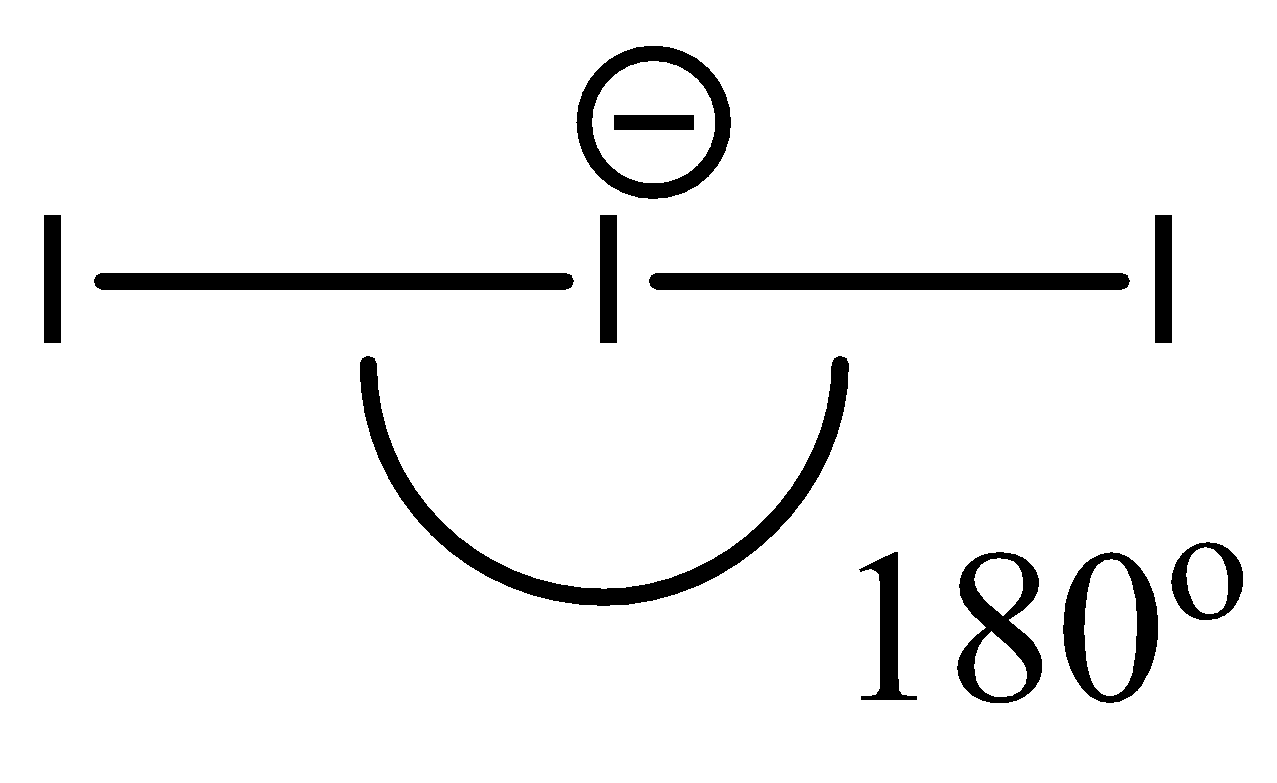
- Since ${{I}_{3}}^{-}$ contains only 3 atoms, despite having an extra electron, its geometry is linear. Hence, the bond angle is around $180{}^\circ $.
Hence, the answer is ‘D. ${{I}_{3}}^{-}$ $>$ $B{{F}_{3}}$ $>$ $N{{H}_{3}}$ $>$ $P{{F}_{3}}$’.
Note:
The bond angle of $P{{F}_{3}}$ is even smaller than $N{{H}_{3}}$. This happens due to the larger size of $F$ as compared to $H$. $F$ also repels the lone pair present on $P$ more strongly than $H$due to its own 3 lone pairs.
Complete step by step answer:
First, let us look at the geometry of all the molecules:
- The geometry of $B{{F}_{3}}$ is trigonal planar. It involves 4 atoms, no lone pairs with a hybridization of $s{{p}^{2}}$. This means that the bond angle between the atoms will be $120{}^\circ $.

- The geometry of $N{{H}_{3}}$ is pyramidal. Technically, the geometry is tetrahedral, but since one of the corners is occupied by a lone pair it is sometimes called pyramidal. It involves 4 atoms, 1 lone pair with a hybridization of $s{{p}^{3}}$. This means that the bond angle will be less than $109{}^\circ $ due to the presence of a lone pair. It is found to be around $107{}^\circ $.

- The geometry of $P{{F}_{3}}$ is again tetrahedral. It involves 4 atoms, 1 lone pair with a hybridization of $s{{p}^{3}}$.

- Since ${{I}_{3}}^{-}$ contains only 3 atoms, despite having an extra electron, its geometry is linear. Hence, the bond angle is around $180{}^\circ $.

Hence, the answer is ‘D. ${{I}_{3}}^{-}$ $>$ $B{{F}_{3}}$ $>$ $N{{H}_{3}}$ $>$ $P{{F}_{3}}$’.
Note:
The bond angle of $P{{F}_{3}}$ is even smaller than $N{{H}_{3}}$. This happens due to the larger size of $F$ as compared to $H$. $F$ also repels the lone pair present on $P$ more strongly than $H$due to its own 3 lone pairs.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




