
Deduce the structure of $Br{{F}_{5}}$ based on the VSEPR theory.
Answer
590.1k+ views
Hint: VSEPR theory provides the postulates for the prediction of the molecules from the electron pairs that surround the central atoms of the molecule. The formula used for the prediction of molecular shape is
$\text{Number of electrons}=\dfrac{1}{2}[V+N-C+A]$
where V is the number of valence electrons in the central atom
N is the number of monovalent atoms bonded to the central atom
C is the charge of the cation
A is the charge of anion.
Complete step by step answer:
-VSEPR theory was first put forward by Sidgwick and Powell in the year 1940.
-VSEPR theory assumes that the molecule will take a shape such that electronic repulsion in the valence shell of that atom is minimized.
-Following are the postulates of the VSEPR (Valence-shell electron-pair repulsion) theory-
(i) In polyatomic molecules, there is one of the atoms among the constituents which is identified as the central atom to which all the other atoms are linked.
(ii) The total number of valence shell electrons pairs present will decide the shape of the molecule.
(iii) The electron pairs tend to orient themselves in such a way that they experience the minimum electron-electron repulsion between them and maximizes the distance between them.
(iv) The valence shell can be thought of as a sphere wherein the electron pairs are localized on the surface in a way that the distance between them is maximized.
(v) Multiple bonds are treated as if it is a single electron pair and the two or three electron pairs of multiple bonds are treated as a single electron pair.
(vi) Where two or more resonance structures can represent a molecule, the VSEPR model applies to only such molecules.
(viii) The repulsive interaction of electron pairs decreases in the following order:
lone pair – lone pair $ > $ lone pair – bond pair $ > $ bond pair – bond pair.
-Let us now predict the structure of $Br{{F}_{5}}$ using the above postulates and the formula,
$\text{Number of electrons}=\dfrac{1}{2}[V+N-C+A]$
Here, V = 7 ; N = 3 ; C = 0 ; A = 0
Inserting these values in the equation gives the number of electrons as 5, which means that the hybridization will be $s{{p}^{3}}d$ and the geometry will be trigonal pyramidal.
-But, due to the presence of three atoms around the central bromine atom, the lone pair of electrons will occupy the fourth and fifth positions. Since the repulsion between the lone pair and bond pair of electrons is more, hence the geometry will be distorted T-shaped.
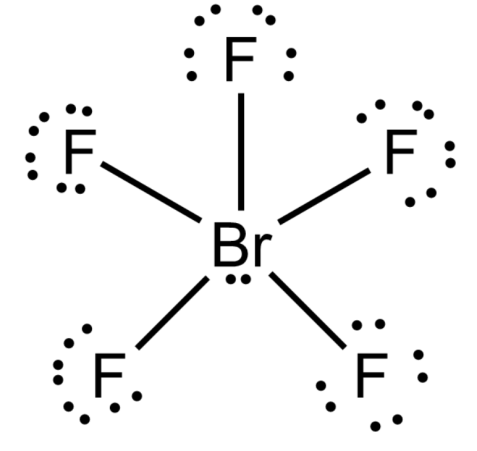
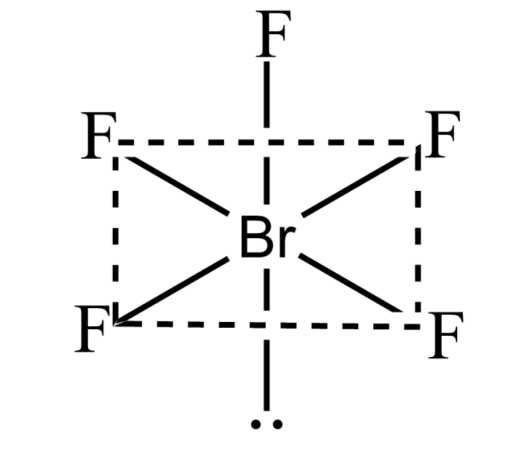
Note: Some significant limitations of VSEPR theory are- it fails to explain isoelectronic species. It failed to predict the structures of transition metal compounds. It made a wrong prediction for the structure of halides of group 2 elements. According to VSEPR theory, it will have a linear shape, but in actuality they have a bent shape.
$\text{Number of electrons}=\dfrac{1}{2}[V+N-C+A]$
where V is the number of valence electrons in the central atom
N is the number of monovalent atoms bonded to the central atom
C is the charge of the cation
A is the charge of anion.
Complete step by step answer:
-VSEPR theory was first put forward by Sidgwick and Powell in the year 1940.
-VSEPR theory assumes that the molecule will take a shape such that electronic repulsion in the valence shell of that atom is minimized.
-Following are the postulates of the VSEPR (Valence-shell electron-pair repulsion) theory-
(i) In polyatomic molecules, there is one of the atoms among the constituents which is identified as the central atom to which all the other atoms are linked.
(ii) The total number of valence shell electrons pairs present will decide the shape of the molecule.
(iii) The electron pairs tend to orient themselves in such a way that they experience the minimum electron-electron repulsion between them and maximizes the distance between them.
(iv) The valence shell can be thought of as a sphere wherein the electron pairs are localized on the surface in a way that the distance between them is maximized.
(v) Multiple bonds are treated as if it is a single electron pair and the two or three electron pairs of multiple bonds are treated as a single electron pair.
(vi) Where two or more resonance structures can represent a molecule, the VSEPR model applies to only such molecules.
(viii) The repulsive interaction of electron pairs decreases in the following order:
lone pair – lone pair $ > $ lone pair – bond pair $ > $ bond pair – bond pair.
-Let us now predict the structure of $Br{{F}_{5}}$ using the above postulates and the formula,
$\text{Number of electrons}=\dfrac{1}{2}[V+N-C+A]$
Here, V = 7 ; N = 3 ; C = 0 ; A = 0
Inserting these values in the equation gives the number of electrons as 5, which means that the hybridization will be $s{{p}^{3}}d$ and the geometry will be trigonal pyramidal.
-But, due to the presence of three atoms around the central bromine atom, the lone pair of electrons will occupy the fourth and fifth positions. Since the repulsion between the lone pair and bond pair of electrons is more, hence the geometry will be distorted T-shaped.


Note: Some significant limitations of VSEPR theory are- it fails to explain isoelectronic species. It failed to predict the structures of transition metal compounds. It made a wrong prediction for the structure of halides of group 2 elements. According to VSEPR theory, it will have a linear shape, but in actuality they have a bent shape.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




