
Define cell constant. Draw a neat and well labeled diagram of a primary reference electrode?
Answer
570.6k+ views
Hint: In the standard hydrogen electrode also known as reference electrode pure and dried hydrogen gas having pressure 1 atm is passed over a platinum plate which is dipped in 1M hydrogen ion solution at 298K. This electrode is used as a reference electrode.
Complete answer:
The cell constant is defined as the ratio of distance between the electrodes which is divided by the area of the cross-sectional of the electrode or we can say that the cell constant is defined as the ratio of distance between the conductance titration electrodes which is measured from the determination resistance of the solution of specific conductance. The unit of cell constant is $c{{m}^{-1}}$ .
Construction of the standard hydrogen electrode is as follows:
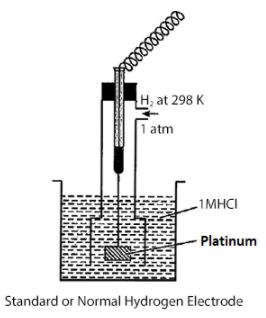
The platinum plate is coated with platinum black to increase the surface area of the platinum plate. Platinized platinum plate is then dipped in 1 M hydrogen ion solution, it has a high surface area which helps to absorb the hydrogen gas.
The reactions which take place on the platinum electrodes is due to its relatively inert nature and the standard oxidation and reduction potential of the standard hydrogen electrode is zero.
Note:
The standard hydrogen electrode has the value of standard cell potential equal to zero. It is also known as the primary reference electrode. Salt bridge is used to connect the standard hydrogen electrode to the other half cells whose electrode potential is unknown.
Complete answer:
The cell constant is defined as the ratio of distance between the electrodes which is divided by the area of the cross-sectional of the electrode or we can say that the cell constant is defined as the ratio of distance between the conductance titration electrodes which is measured from the determination resistance of the solution of specific conductance. The unit of cell constant is $c{{m}^{-1}}$ .
Construction of the standard hydrogen electrode is as follows:

The platinum plate is coated with platinum black to increase the surface area of the platinum plate. Platinized platinum plate is then dipped in 1 M hydrogen ion solution, it has a high surface area which helps to absorb the hydrogen gas.
The reactions which take place on the platinum electrodes is due to its relatively inert nature and the standard oxidation and reduction potential of the standard hydrogen electrode is zero.
Note:
The standard hydrogen electrode has the value of standard cell potential equal to zero. It is also known as the primary reference electrode. Salt bridge is used to connect the standard hydrogen electrode to the other half cells whose electrode potential is unknown.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




