
Define isoelectric point of amino acid. The isoelectric point of alanine is 6.1.write the molecular form of alanine at \[pH\] 5.
Answer
577.2k+ views
Hint: The \[pH\] at which there is no net migration of amino acid under the influence of applied electric field is called isoelectric point. \[pH\] 5 that is acidic medium and in acidic medium amino acid goes towards cathode.
Complete step by step answer: Amino acid contains both acidic and basic groups that are carboxyl group and amino group respectively. These two groups interact with each other and there is transfer of proton from carboxyl group to amino group within the molecule.as a result amino acid exists as a dipolar ion. Which is called zwitterion.
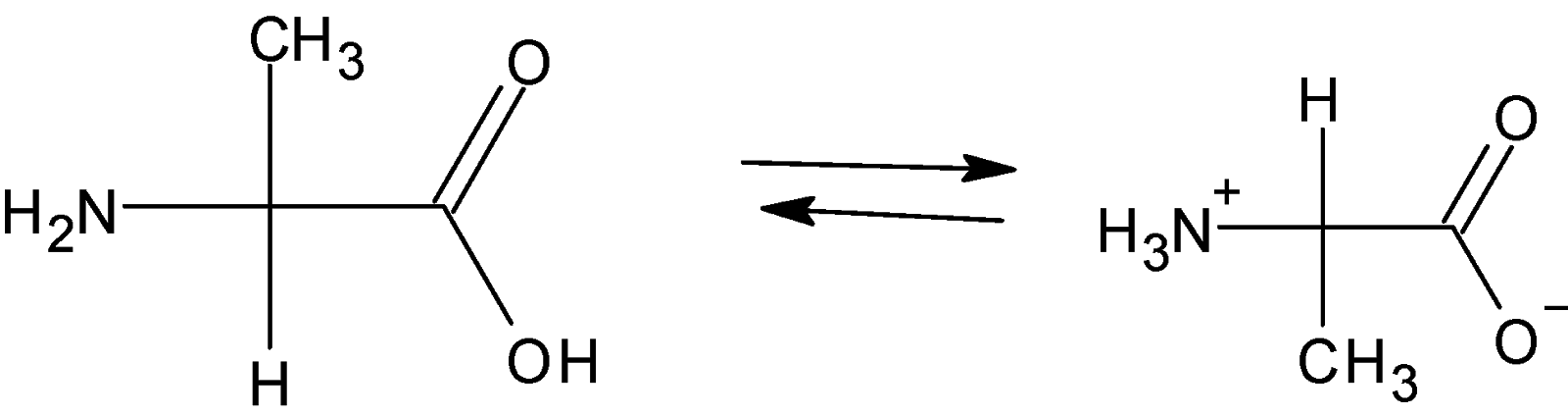
In acidic medium amino acids exist as a cation and thus migrate towards cathode under the influence of electric field. On other hand in alkaline medium amino acid exist as anion hence migrate towards anode under the influence of electric field.at some intermediate value of \[pH\]concentration of cationic and anionic form are equal and hence amino acid exist as a dipolar ion that is it contains both cation and anion (zwitter ionic form) at this \[pH\]there is no net migration of amino acid in an electric field. Therefore,That \[pH\]at which there is no net migration of amino acid under the influence of applied electric field is called isoelectric point.
Given: the isoelectric point of alanine is 6.1
So, below isoelectric point i.e. \[pH\]= 5 is the acidic medium and as we discussed above in acidic medium amino acid exists as cation.
Therefore molecular form of alanine at \[pH\]= 5 is
Note:
For neutral amino acid isoelectric point is slightly less than 7
For acidic amino acid isoelectric point is lies between 3 - 5.4
For basic amino acid isoelectric point lies between 7.6 - 10.8.
At isoelectric point amino acid has least solubility in water and hence this property has been used in the separation of different amino acids obtained from hydrolysis of protein.
Complete step by step answer: Amino acid contains both acidic and basic groups that are carboxyl group and amino group respectively. These two groups interact with each other and there is transfer of proton from carboxyl group to amino group within the molecule.as a result amino acid exists as a dipolar ion. Which is called zwitterion.

In acidic medium amino acids exist as a cation and thus migrate towards cathode under the influence of electric field. On other hand in alkaline medium amino acid exist as anion hence migrate towards anode under the influence of electric field.at some intermediate value of \[pH\]concentration of cationic and anionic form are equal and hence amino acid exist as a dipolar ion that is it contains both cation and anion (zwitter ionic form) at this \[pH\]there is no net migration of amino acid in an electric field. Therefore,That \[pH\]at which there is no net migration of amino acid under the influence of applied electric field is called isoelectric point.
Given: the isoelectric point of alanine is 6.1
So, below isoelectric point i.e. \[pH\]= 5 is the acidic medium and as we discussed above in acidic medium amino acid exists as cation.
Therefore molecular form of alanine at \[pH\]= 5 is
Note:
For neutral amino acid isoelectric point is slightly less than 7
For acidic amino acid isoelectric point is lies between 3 - 5.4
For basic amino acid isoelectric point lies between 7.6 - 10.8.
At isoelectric point amino acid has least solubility in water and hence this property has been used in the separation of different amino acids obtained from hydrolysis of protein.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




