
Define nuclear fission.
Answer
585.3k+ views
Hint:The reaction that includes the change in the identity in the personality of the atomic nucleus, initiated by bombarding it with a high energy molecule. The bombarding molecule may either be an alpha molecule, a gamma-ray photon, a neutron, a proton, or a heavy particle. Regardless, the bombarding molecule must have enough energy to move toward the positively charged nucleus to inside the range of the solid atomic power.
Complete step by step solution:
In general, there are four major and main types of the nuclear reaction. They are nuclear fission, nuclear fusion, nuclear decay and transmutation. In nuclear reaction, when the nucleus of an atom splits into lighter nuclei through a nuclear reaction, then the process is termed as nuclear fission. This decay can be natural spontaneous splitting by radioactive decay, or can actually be simulated in a lab by achieving necessary conditions (bombarding with neutrinos). The resulting fragments tend to have a combined mass which is less than the original. The missing mass is what is converted into nuclear energy in the above reaction.
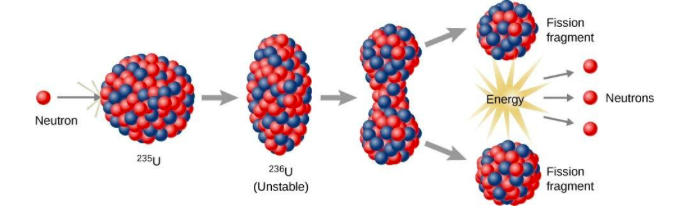
In other terms, when the nucleus of the atom of a heavy radioactive particle is split up into two lighter nuclei with the release of a large amount of energy. This reaction is used in the atom bomb, where the uranium is broken into two pieces with a large amount of release of the energy.
Note: Nuclear fission reaction produces the atomic bomb, a weapon of mass destruction that uses the power released by the splitting of atomic nuclei. When a single free neutron strikes the nucleus of an atom of radioactive material like uranium or plutonium, it knocks two or three more neutrons free.
Complete step by step solution:
In general, there are four major and main types of the nuclear reaction. They are nuclear fission, nuclear fusion, nuclear decay and transmutation. In nuclear reaction, when the nucleus of an atom splits into lighter nuclei through a nuclear reaction, then the process is termed as nuclear fission. This decay can be natural spontaneous splitting by radioactive decay, or can actually be simulated in a lab by achieving necessary conditions (bombarding with neutrinos). The resulting fragments tend to have a combined mass which is less than the original. The missing mass is what is converted into nuclear energy in the above reaction.

In other terms, when the nucleus of the atom of a heavy radioactive particle is split up into two lighter nuclei with the release of a large amount of energy. This reaction is used in the atom bomb, where the uranium is broken into two pieces with a large amount of release of the energy.
Note: Nuclear fission reaction produces the atomic bomb, a weapon of mass destruction that uses the power released by the splitting of atomic nuclei. When a single free neutron strikes the nucleus of an atom of radioactive material like uranium or plutonium, it knocks two or three more neutrons free.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




