
Define representative elements.
Answer
524.4k+ views
Hint: The modern periodic table is the representation of the elements. The elements are arranged in the increasing order of their atomic numbers. There are 4 blocks on the periodic table s, p, d, and f blocks. The representative elements are placed in the 2 major blocks of the periodic table or the main group elements.
Complete answer:
The modern periodic table is based on the modern periodic law that states, physical and chemical properties of elements are the periodic function of their atomic numbers. This means the elements in the modern periodic table are arranged according to the increasing order of their atomic number. The 4 blocks in the periodic table are s, p, d, and f that have varying properties of elements.
Representative elements are the elements placed in the s and p block. These elements consist of the groups 1, 2, 13, 14, 15, 16 and 17. This is because that the outer shells of the elements in these blocks are not completely filled, they have valence shell configuration as $n{{s}^{1-2}}$ for s block and $n{{p}^{1-5}}$ for p block. This makes them reactive and names them representative elements. The representative elements can be seen on the periodic table from groups 1 to 2 and from 13 to 17 as follows:
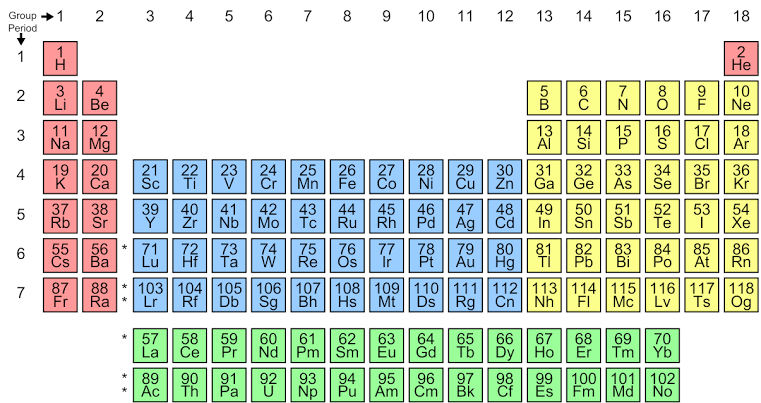
Hence, representative elements are defined as the elements of the s and p block (except group 18) of the periodic table, which are from group 1 to 2 and 13 to 17.
Note:
The noble gases (group 18) do not come under representative elements as they have a fully filled configuration and they are unreactive in nature. The d block elements are called as transition elements as they have properties transiting from metals and non – metals. While the f block elements are called inner – transition elements that consist of lanthanides and actinides.
Complete answer:
The modern periodic table is based on the modern periodic law that states, physical and chemical properties of elements are the periodic function of their atomic numbers. This means the elements in the modern periodic table are arranged according to the increasing order of their atomic number. The 4 blocks in the periodic table are s, p, d, and f that have varying properties of elements.
Representative elements are the elements placed in the s and p block. These elements consist of the groups 1, 2, 13, 14, 15, 16 and 17. This is because that the outer shells of the elements in these blocks are not completely filled, they have valence shell configuration as $n{{s}^{1-2}}$ for s block and $n{{p}^{1-5}}$ for p block. This makes them reactive and names them representative elements. The representative elements can be seen on the periodic table from groups 1 to 2 and from 13 to 17 as follows:

Hence, representative elements are defined as the elements of the s and p block (except group 18) of the periodic table, which are from group 1 to 2 and 13 to 17.
Note:
The noble gases (group 18) do not come under representative elements as they have a fully filled configuration and they are unreactive in nature. The d block elements are called as transition elements as they have properties transiting from metals and non – metals. While the f block elements are called inner – transition elements that consist of lanthanides and actinides.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




