
Define the following terms:
(i) Lyophilic colloid
(ii) Zeta potential
(iii) Associated colloids
Answer
592.2k+ views
Hint: In colloidal state of matter, size of the particles ranges from 1 nm to 100 nm (${{10}^{-9}}$ to ${{10}^{-7}}$ m). Colloidal particles can pass through filter paper but not through plasma or animal membrane. In a colloidal system, the substance which is distributed in a medium in the form of colloidal particles is called the dispersed phase and the medium in which it is dispersed is called the dispersion medium.
Complete answer:
(i) Various substances when dissolved in suitable dispersion medium form colloids. The colloids formed are classified into lyophilic and lyophobic colloids.
Lyophilic colloid: Lyophilic means liquid loving. Substances which when mixed with a suitable liquid as dispersion medium directly results in the formation of the colloidal sol (sols have solid dispersed phase and liquid dispersion medium) are called lyophilic colloids. For example, organic substances like gum, starch, rubber, gelatin, etc. The colloidal sol formed is called lyophilic sol.
They are also called intrinsic colloids as they form colloidal sol directly.
Lyophilic sols are reversible in nature, i.e. if the dispersed phase and dispersion medium are separated, the sol can be formed by remixing the dispersed phase with the dispersion medium.
These colloid sols are stable and cannot be coagulated or precipitated easily.
(ii) Zeta potential: Stability of a colloidal sol is due to the presence of charge on the colloidal particles. All the dispersed particles in a colloidal sol carry the same charge, they repel each other and hence, do not form large particles or aggregates. Charge on the dispersion medium is an equal and opposite of that on the dispersed particles.
The origin of charge on the colloidal particles is explained by the preferential adsorption of one type of ions from the electrolyte.
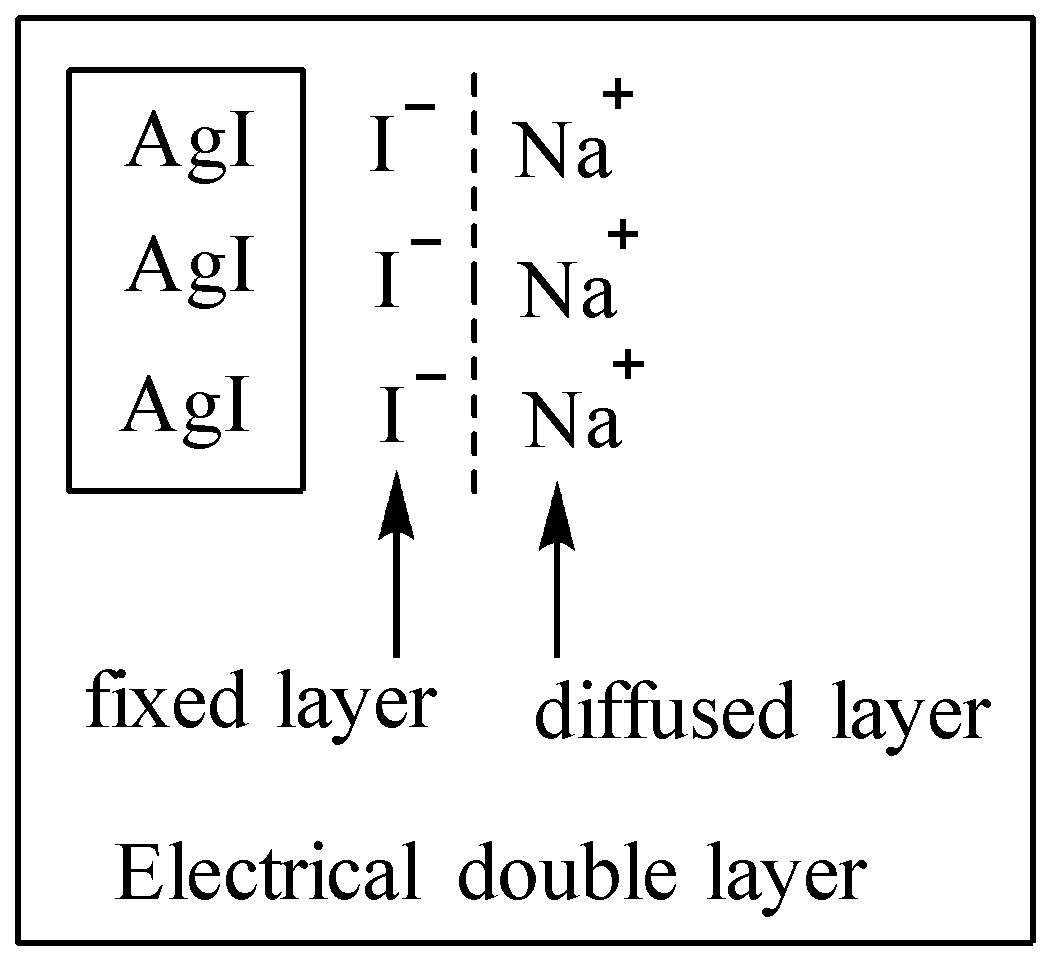
When one type of common ion is adsorbed on the surface of colloidal particles, a fixed layer is formed. This fixed layer then attracts the counter ions from the medium. The counter ions form the diffused layer. Consequently, a potential difference exists between the fixed layer and the diffused layer. This potential difference is zeta potential or electro kinetic potential.
For example: let us assume that in the formation of silver iodide (AgI) from silver nitrate ($AgN{{O}_{3}}$) and sodium iodide (NaI), the electrolyte NaI is used in excess.
\[AgN{{O}_{3}}(aq)+NaI\to AgI\downarrow +NaN{{O}_{3}}\]
The AgI will preferentially adsorb ${{I}^{-}}$ ion, resulting in the negative charge on them. Due to their negative charge the counter positive ions, i.e. $N{{a}^{+}}$ ions are attracted.
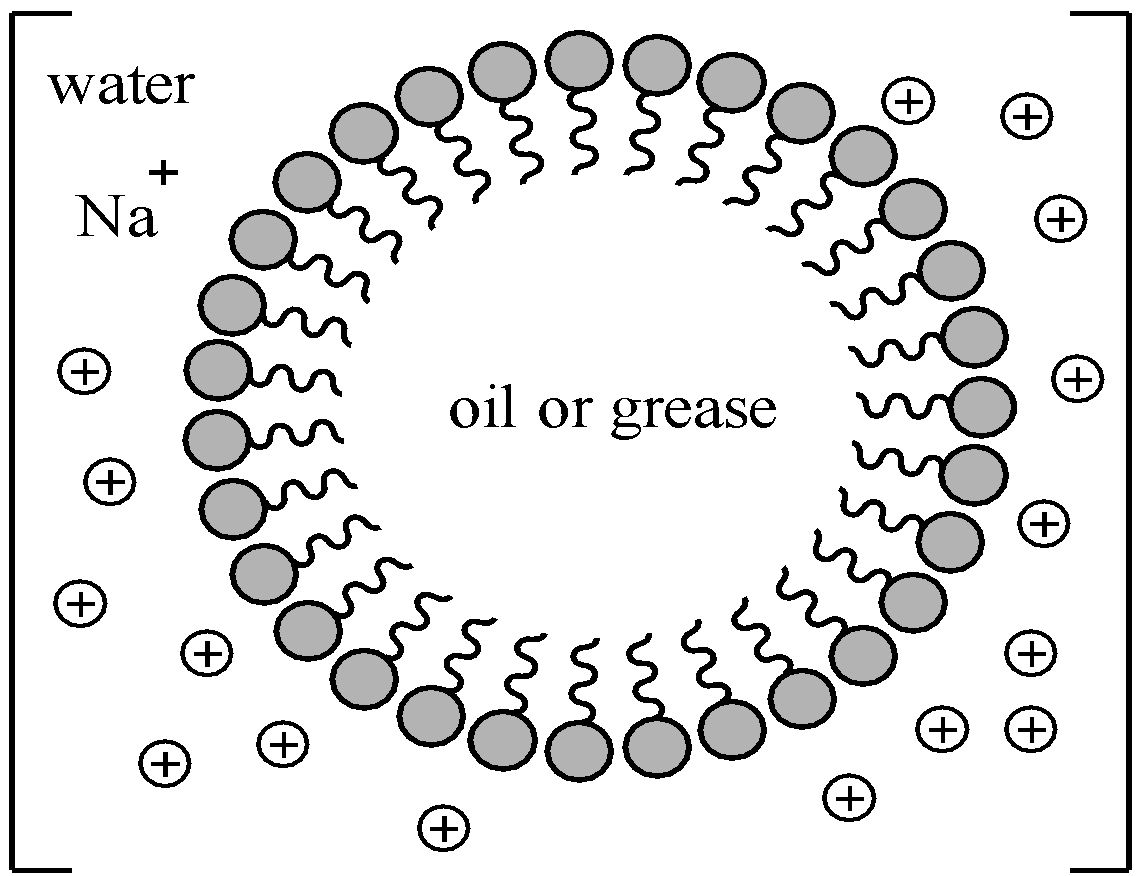
(iii) Associated colloids: Substances when dissolved in a medium at low concentrations behave as normal electrolytes but at higher concentration aggregate to form particles which have sizes in the colloidal range are called as associated colloids. The narrow concentration range over which the physicochemical properties of particles change due to the formation of oriented colloidal aggregates is called critical micelle concentration (CMC) and the aggregates formed are called micelles. For example: surface active agents like soaps and detergents. The formation of micelle takes place at a particular temperature called as Kraft temperature ${{T}_{k}}$
Note: Colloids are classified into lyophilic and lyophobic based on the substances forming the colloids and they are classified into multimolecular, macromolecular and associated colloids based on the how different substances have size in the colloidal range. Multi means many so, in multimolecular many different particles combine to give a colloidal size particle whereas in macromolecular colloids, individual particles have colloidal size.
Complete answer:
(i) Various substances when dissolved in suitable dispersion medium form colloids. The colloids formed are classified into lyophilic and lyophobic colloids.
Lyophilic colloid: Lyophilic means liquid loving. Substances which when mixed with a suitable liquid as dispersion medium directly results in the formation of the colloidal sol (sols have solid dispersed phase and liquid dispersion medium) are called lyophilic colloids. For example, organic substances like gum, starch, rubber, gelatin, etc. The colloidal sol formed is called lyophilic sol.
They are also called intrinsic colloids as they form colloidal sol directly.
Lyophilic sols are reversible in nature, i.e. if the dispersed phase and dispersion medium are separated, the sol can be formed by remixing the dispersed phase with the dispersion medium.
These colloid sols are stable and cannot be coagulated or precipitated easily.
(ii) Zeta potential: Stability of a colloidal sol is due to the presence of charge on the colloidal particles. All the dispersed particles in a colloidal sol carry the same charge, they repel each other and hence, do not form large particles or aggregates. Charge on the dispersion medium is an equal and opposite of that on the dispersed particles.
The origin of charge on the colloidal particles is explained by the preferential adsorption of one type of ions from the electrolyte.
When one type of common ion is adsorbed on the surface of colloidal particles, a fixed layer is formed. This fixed layer then attracts the counter ions from the medium. The counter ions form the diffused layer. Consequently, a potential difference exists between the fixed layer and the diffused layer. This potential difference is zeta potential or electro kinetic potential.
For example: let us assume that in the formation of silver iodide (AgI) from silver nitrate ($AgN{{O}_{3}}$) and sodium iodide (NaI), the electrolyte NaI is used in excess.
\[AgN{{O}_{3}}(aq)+NaI\to AgI\downarrow +NaN{{O}_{3}}\]
The AgI will preferentially adsorb ${{I}^{-}}$ ion, resulting in the negative charge on them. Due to their negative charge the counter positive ions, i.e. $N{{a}^{+}}$ ions are attracted.

(iii) Associated colloids: Substances when dissolved in a medium at low concentrations behave as normal electrolytes but at higher concentration aggregate to form particles which have sizes in the colloidal range are called as associated colloids. The narrow concentration range over which the physicochemical properties of particles change due to the formation of oriented colloidal aggregates is called critical micelle concentration (CMC) and the aggregates formed are called micelles. For example: surface active agents like soaps and detergents. The formation of micelle takes place at a particular temperature called as Kraft temperature ${{T}_{k}}$

Note: Colloids are classified into lyophilic and lyophobic based on the substances forming the colloids and they are classified into multimolecular, macromolecular and associated colloids based on the how different substances have size in the colloidal range. Multi means many so, in multimolecular many different particles combine to give a colloidal size particle whereas in macromolecular colloids, individual particles have colloidal size.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




