
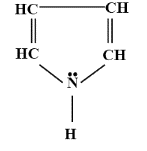
How many delocalised $\pi $-electrons are there in the following compound ?
A. 1
B. 2
C. 4
D. 6

Answer
592.2k+ views
Hint: A pair of electrons which do not make any bond are known as lone pairs whereas a pair of electrons, which is found in the paired condition they are known as electron pairs. Lone pairs electrons can move in the lattice structure.
Complete step by step answer:
- The delocalised electrons are those electrons which can move from one bond to the other in a molecule structure.
- The structure which is given in the question is of pyrroles, a 5-membered ring.
- In the given, we have to tell about the delocalised electron but they should belong to $\pi $- electrons.
- $\pi $-electrons are those electrons which are formed when a double-bond is formed i.e. they are found in the double-bond.
- So, as we can see that there are a total of two double bonds so there are 4 $\pi $-electrons.
- Along with it, two delocalised $\pi $-electrons are also present above the nitrogen atom.
- So, a total of 6 delocalised $\pi $- electrons are present in the pyrrole.
- These delocalised electrons are also responsible for the electrical conduction due to their movement in the lattice structure.
Therefore, option D. is the correct answer.
Note: $\pi $-electrons are found in the double bond whereas $\sigma $- electrons are found in the single bond. Usually without the formation of sigma bond the formation of $\pi $bond can not take place. Moreover, benzene is another example of the delocalised $\pi $-electron due to which the benzene has a stable structure.
Complete step by step answer:
- The delocalised electrons are those electrons which can move from one bond to the other in a molecule structure.
- The structure which is given in the question is of pyrroles, a 5-membered ring.
- In the given, we have to tell about the delocalised electron but they should belong to $\pi $- electrons.
- $\pi $-electrons are those electrons which are formed when a double-bond is formed i.e. they are found in the double-bond.
- So, as we can see that there are a total of two double bonds so there are 4 $\pi $-electrons.
- Along with it, two delocalised $\pi $-electrons are also present above the nitrogen atom.
- So, a total of 6 delocalised $\pi $- electrons are present in the pyrrole.
- These delocalised electrons are also responsible for the electrical conduction due to their movement in the lattice structure.
Therefore, option D. is the correct answer.
Note: $\pi $-electrons are found in the double bond whereas $\sigma $- electrons are found in the single bond. Usually without the formation of sigma bond the formation of $\pi $bond can not take place. Moreover, benzene is another example of the delocalised $\pi $-electron due to which the benzene has a stable structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




