
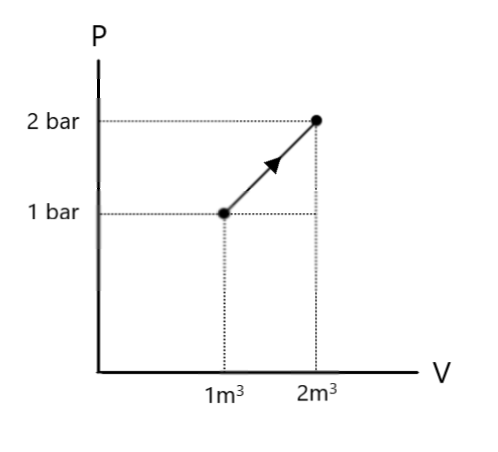
What is $\Delta U$ for the process described by figure. Heat supplied during the process $q = 100kJ$.
A. $ + 50kJ$
B. $ - 50kJ$
C. $ - 150kJ$
D. $ + 150kJ$

Answer
569.1k+ views
Hint: We must first find the work done by the gas by evaluating the area under the $P - V$ diagram. Since the change in internal energy is the difference between the heat supplied and the work done, we can get the answer using the mathematical expression of the first law of thermodynamics.
Formulas used: $\Delta U = q + W$
Where $\Delta U$ is the change in internal energy, $q$ is the heat supplied and $W$ is the work done by the gas.
Area of triangle $ = \dfrac{1}{2}bh$
Where $b$ is the base of the triangle and $h$ is its height.
Area of rectangle $ = lb$
Where
$l$ is its length and $b$ is its breadth.
Complete step by step answer:
According to the First Law of Thermodynamics, the heat supplied to a gas is used to do work, and to raise the internal energy of the system. Thus, we have:
$\Delta U = q + W$
Where $\Delta U$ is the change in internal energy, $q$ is the heat supplied and $W$ is the work done by the gas.
The work done, as we know, is obtained by evaluating the area under the $P - V$ diagram. Looking at the $P - V$ diagram given in question, we find that the area required is actually the sum of areas of a triangle and a rectangle.
As we know, Area of triangle $ = \dfrac{1}{2}bh$
Where $b$ is the base of the triangle and $h$ is its height.
As we can see from the diagram, the height of the triangle is $(2 - 1) = 1bar$ and its base is $(2 - 1) = 1{m^3}$. Hence, we have:
Area of triangle $ = \dfrac{1}{2} \times 1 \times 1 = 0.5bar.{m^3}$
Area of rectangle $ = lb$
Where $l$ is its length and $b$ is its breadth.
Here, from the diagram, we see that the length of the rectangle is $1bar$ and its breadth is $(2 - 1) = 1{m^3}$. Hence, we have:
Area of rectangle $ = 1 \times 1 = 1bar.{m^3}$
Hence, the total area is the sum of areas of the triangle and the rectangle, which is:
$ = 0.5 + 1 = 1.5bar.{m^3}$
We need to convert this into SI units for ease of calculation. As we know, $1bar = {10^5}Pa = {10^5}N/{m^2}$. Hence, we have:
Work done = area under the graph $ = 1.5 \times {10^5}(N/{m^2}){m^3} = 1.5 \times {10^5}Nm$.
This is now in SI units, in which the unit of energy is Joules. Thus, we have:
$W = 1.5 \times {10^5}J = 1.5 \times {10^2}kJ$
$ \Rightarrow W = 150kJ$
Since in chemistry, the convention is to put the work done by a gas as negative,
$W = - 150kJ$
Substituting this and $q = 100kJ$ in the first law equation, we have:
$\Delta U = 100 - 150 = - 50kJ$
Hence, the internal energy change for this process is $ - 50kJ$.
So, the correct answer is Option B.
Note: In chemistry, the convention is to put heat supplied and work done on a gas as positive, and heat released and work done by the gas as negative. Note that the process undergone here is expansion, since from the figure, we see that the volume is increasing. Thus, the gas has utilised the heat supplied to do some work (expansion) and to change its internal energy.
Formulas used: $\Delta U = q + W$
Where $\Delta U$ is the change in internal energy, $q$ is the heat supplied and $W$ is the work done by the gas.
Area of triangle $ = \dfrac{1}{2}bh$
Where $b$ is the base of the triangle and $h$ is its height.
Area of rectangle $ = lb$
Where
$l$ is its length and $b$ is its breadth.
Complete step by step answer:
According to the First Law of Thermodynamics, the heat supplied to a gas is used to do work, and to raise the internal energy of the system. Thus, we have:
$\Delta U = q + W$
Where $\Delta U$ is the change in internal energy, $q$ is the heat supplied and $W$ is the work done by the gas.
The work done, as we know, is obtained by evaluating the area under the $P - V$ diagram. Looking at the $P - V$ diagram given in question, we find that the area required is actually the sum of areas of a triangle and a rectangle.
As we know, Area of triangle $ = \dfrac{1}{2}bh$
Where $b$ is the base of the triangle and $h$ is its height.
As we can see from the diagram, the height of the triangle is $(2 - 1) = 1bar$ and its base is $(2 - 1) = 1{m^3}$. Hence, we have:
Area of triangle $ = \dfrac{1}{2} \times 1 \times 1 = 0.5bar.{m^3}$
Area of rectangle $ = lb$
Where $l$ is its length and $b$ is its breadth.
Here, from the diagram, we see that the length of the rectangle is $1bar$ and its breadth is $(2 - 1) = 1{m^3}$. Hence, we have:
Area of rectangle $ = 1 \times 1 = 1bar.{m^3}$
Hence, the total area is the sum of areas of the triangle and the rectangle, which is:
$ = 0.5 + 1 = 1.5bar.{m^3}$
We need to convert this into SI units for ease of calculation. As we know, $1bar = {10^5}Pa = {10^5}N/{m^2}$. Hence, we have:
Work done = area under the graph $ = 1.5 \times {10^5}(N/{m^2}){m^3} = 1.5 \times {10^5}Nm$.
This is now in SI units, in which the unit of energy is Joules. Thus, we have:
$W = 1.5 \times {10^5}J = 1.5 \times {10^2}kJ$
$ \Rightarrow W = 150kJ$
Since in chemistry, the convention is to put the work done by a gas as negative,
$W = - 150kJ$
Substituting this and $q = 100kJ$ in the first law equation, we have:
$\Delta U = 100 - 150 = - 50kJ$
Hence, the internal energy change for this process is $ - 50kJ$.
So, the correct answer is Option B.
Note: In chemistry, the convention is to put heat supplied and work done on a gas as positive, and heat released and work done by the gas as negative. Note that the process undergone here is expansion, since from the figure, we see that the volume is increasing. Thus, the gas has utilised the heat supplied to do some work (expansion) and to change its internal energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




