
Derive an expression for the height in capillary rise between two parallel plates dipping in a liquid of density ‘$\sigma $’ separated by a distance ‘d’. The surface tension of the liquid is T.
Answer
526.8k+ views
Hint: We will calculate the pressure at two different heights in the capillary tube and then use it to derive an expression for the rise in height of liquid in the capillary tube. Also, since nothing is mentioned about the angle of contact of the liquid with the surface, we will take the angle of contact of the liquid and the surface as zero degree.
Complete answer:
Let us first try to understand our problem with the help of the following diagram:
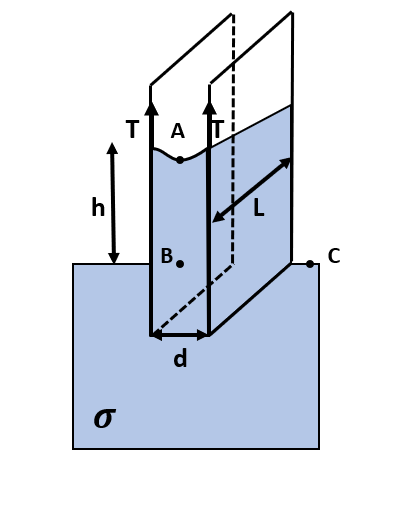
Here, we assume the rise in height of water in the capillary tube is ‘h’ and the length of the two sheets is equal to ‘L’. Also, let the net force due to surface tension be ‘${{F}_{T}}$’.
Also, since the angle of contact of the water and the capillary tube is zero, the force due to surface tension will act vertically upwards at the two ends.
Now, the force of surface tension at the contact of the lengths of the two sheets is equal to:
$\begin{align}
& \Rightarrow {{F}_{T}}=2\times \left[ T\times L\times \cos \left( {{0}^{\circ }} \right) \right] \\
& \Rightarrow {{F}_{T}}=2TL \\
\end{align}$
This force is acting upwards and should be balanced by the weight of liquid starting from point A to point B, where point B is at the same height as point C, that is, the top of the container.
This weight of water (say, W) will be equal to:
$\begin{align}
& \Rightarrow W=\left( \text{Volume of water rises} \right)\times \left( \sigma \right)\times \left( g \right) \\
& \Rightarrow W=\left( d\times l\times h \right)\times \left( \sigma \right)\times \left( g \right) \\
& \Rightarrow W=dlh\sigma g \\
\end{align}$
Where, ‘g’ is the acceleration due to gravity.
Now, equating this weight of water with the force due to surface tension, we get:
$\begin{align}
& \Rightarrow dLh\sigma g=2TL \\
& \therefore h=\dfrac{2T}{\sigma dg} \\
\end{align}$
Hence, the expression for the height in capillary rise between two parallel plates dipping in a liquid of density ‘$\sigma $’ separated by a distance ‘d’ and having surface tension ‘T’ comes out to be, $h=\dfrac{2T}{\sigma dg}$.
Note:
While solving problems like this, we should be sure of our approach. Also, whenever the contact angle of a liquid and a solid is not mentioned in a problem, we will take it as zero. This is a standard assumption and can be applied to other questions also.
Complete answer:
Let us first try to understand our problem with the help of the following diagram:

Here, we assume the rise in height of water in the capillary tube is ‘h’ and the length of the two sheets is equal to ‘L’. Also, let the net force due to surface tension be ‘${{F}_{T}}$’.
Also, since the angle of contact of the water and the capillary tube is zero, the force due to surface tension will act vertically upwards at the two ends.
Now, the force of surface tension at the contact of the lengths of the two sheets is equal to:
$\begin{align}
& \Rightarrow {{F}_{T}}=2\times \left[ T\times L\times \cos \left( {{0}^{\circ }} \right) \right] \\
& \Rightarrow {{F}_{T}}=2TL \\
\end{align}$
This force is acting upwards and should be balanced by the weight of liquid starting from point A to point B, where point B is at the same height as point C, that is, the top of the container.
This weight of water (say, W) will be equal to:
$\begin{align}
& \Rightarrow W=\left( \text{Volume of water rises} \right)\times \left( \sigma \right)\times \left( g \right) \\
& \Rightarrow W=\left( d\times l\times h \right)\times \left( \sigma \right)\times \left( g \right) \\
& \Rightarrow W=dlh\sigma g \\
\end{align}$
Where, ‘g’ is the acceleration due to gravity.
Now, equating this weight of water with the force due to surface tension, we get:
$\begin{align}
& \Rightarrow dLh\sigma g=2TL \\
& \therefore h=\dfrac{2T}{\sigma dg} \\
\end{align}$
Hence, the expression for the height in capillary rise between two parallel plates dipping in a liquid of density ‘$\sigma $’ separated by a distance ‘d’ and having surface tension ‘T’ comes out to be, $h=\dfrac{2T}{\sigma dg}$.
Note:
While solving problems like this, we should be sure of our approach. Also, whenever the contact angle of a liquid and a solid is not mentioned in a problem, we will take it as zero. This is a standard assumption and can be applied to other questions also.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




